Childhood Vaccine Schedule
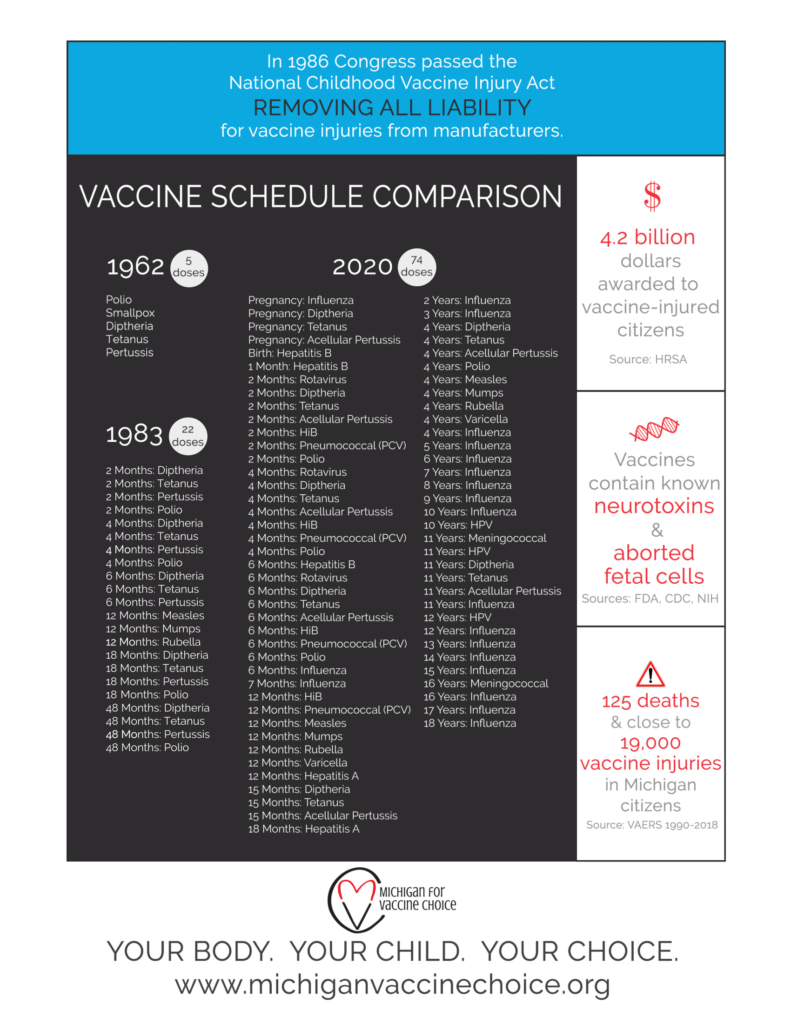
Since the 1986 Childhood Vaccine Injury Act, manufacturers have had no liability for any injury or death resulting from a vaccine that is on the CDC recommended schedule. Due to this, the Childhood Vaccine Schedule has exploded, making it challenging for parents to sift through the ever expanding number of vaccines to try and understand them and the illnesses they are supposed to protect from. Below, I offer an overview of each type of vaccine and the pros and cons associated with it. If you scroll further down, I also offer what the most likely mechanism of injury associated with each type of vaccine.

Learn More About Vaccines on the Childhood Schedule
Medical Disclaimer: The information at vi-ta.org is presented for informational purposes only. Nothing in this website constitutes medical advice and is not intended to diagnose or prescribe for any medical or psychological condition, nor to prevent, treat, mitigate or cure such conditions. Parents, caregivers, consumers, patients, individuals and health practitioners must use their own judgment concerning specific treatment options.
vi-ta.org authors, editors, principals, partners and affiliates disclaim any liability or responsibility to any person or organization for any loss, damage, expense, fine, injury, or penalty that may arise or result from the use of any information, recommendations, opinions and/or errors on this website or in our articles, emails or links. Any use of, or reliance on, information reflected on this website, emails, articles or references is solely the responsibility of the viewer.
vi-ta.org encourages you to make your own health care decisions based on your judgment and research in partnership with a qualified healthcare professional. These statements have not been evaluated by the Food and Drug Administration. The information on this website is not intended to diagnose, treat, cure or prevent any disease.
WHAT DOES IT MEAN TO BE “FULLY VACCINATED”??
- From 5 doses in 1962 to 74 doses in 2020
- The childhood vaccine schedule blossomed after the Vaccine Injury Act was passed in 1986 that removed liability from manufacturers for any vaccine on the CDC recommended childhood schedule.
Conjugate Vaccines
While most vaccines are made to provide immunity to viruses, conjugate vaccines are made in a special way to help the infant body to generate some immunity to bacterial illnesses.
How Are They Made?
Infant immune systems do not respond well to sugars from the outer coat of bacteria. To improve the immune response, these sugars are purified and bound to a toxin (tetanus or diptheria) or protein (Neisseria). Some brands also add an aluminum adjuvant to further improve immune response.
How Are They Made?
Infant immune systems do not respond well to sugars from the outer coat of bacteria. To improve the immune response, these sugars are purified and bound to a toxin (tetanus or diptheria) or protein (Neisseria). Some brands also add an aluminum adjuvant to further improve immune response.
Pros
- Able to prevent life-threatening bacterial meningitis and pneumonia.
Cons
- New strains of bacteria are evolving that are not covered by the vaccine.
- Immunity is often short-lived.
- Fail to activate cellular immunity.
- Often contain aluminum, which is a known neurotoxin.
Bacterial meningitis can be a life-threatening illness. It occurs when pathogenic bacteria set up an infection in the membranes of the brain and/or spinal cord. In recent years, it has become clear that the maintenance of a healthy microbiome is key to keeping the tiny populations of pathogenic bacteria (which can and do inhabit the nose and throat of many healthy people) from proliferating and setting up an infection. While vaccination can potentially reduce the risk of infant bacterial infection, it is important to understand how the infant immune system is designed and why. The reason that vaccines against bacteria include the diphtheria toxin as well as aluminum is because the infant immune system is naturally under-reactive to the presence of bacteria (or its sugars in the case of the vaccine). Thus, the presence of bacteria often does not induce an inflammatory response in infants. It is possible that the infant immune systems is designed this way in order to keep the body in an anti-inflammatory state during critical times of brain development, as high levels of inflammation can negatively affect brain development. To compensate for the under-responsiveness of the infant immune system to bacteria, breastmilk contains many antimicrobial components as well as antibodies against bacteria that are circulating in the environment. Thus, breastfed children have much lower incidence of bacterial infection than their bottle-fed counterparts.
There are two different types of bacteria that commonly cause bacterial meningitis, Haemophilus influenzae and Neisseria meningitidis.
- Haemophilus influenzae more commonly causes meningitis in infants who have never been exposed to these bacteria and in the elderly, whose immune systems are compromised. The HiB vaccine is given to infants to prevent infection with serotype B of the H. influenzae bacteria. Vaccination with HiB did result in less cases of type B meningitis. However, unfortunately, now other H. influenzae strains for which there are no vaccines are the ones that are primarily causing illness. In 2021, the CDC reported 43 cases of H. influenzae illness in children aged 0-4 years. Seven of those 43 cases were serotype B. Thus, it is important to realize that the HiB vaccine does not provide protection from the other strains of H. influenzae currently in circulation.
- Neisseria meningitidis bacteria also causes meningitis in both infants and college-aged persons at rates higher than in the general population. Vaccines against Neisseria meningitidis historically have been given only to college-age persons, but are now being approved (but not yet “recommended”) for infants as young as 9 months. Currently, for children ages 11-12 yrs. and 16 yrs , the CDC recommends meningitis vaccines made to protect against the A, C, Y, W serogroups of the bacteria, including PENBRAYA (which also covers serotype B). In infants, 60% of cases of N. meningitidis are now caused by serogroup B bacteria. However, PENBRAYA and BEXSERO (which is made only against serotype B) are currently only approved for persons aged 10-25 years. BEXSERO contains an alarming 1,500 micrograms of aluminum.
The Illness: Haemophilus Influenza is a bacterial infection that is transmitted through contact with infected person’s cough, mucus, or saliva. The bacteria usually remains in the nose, ears, or throat and causes minor cold symptoms. Although infrequent, it can cause meningitis, blood infections, bone infections, and pneumonia. Severe cases of HiB are diagnosed through a blood test or spinal tap. The treatment for severe infection is intravenous antibiotics.
Cost/Benefit Analysis: Infant bacterial meningitis is a serious, life-threatening condition, but extremely rare at about 25 cases per year. Breastfed infants are at lower risk than bottle-fed infants. HiB vaccines have one of the best safety profiles of all vaccines. PedVaxHIB also contains 225 micrograms of aluminum and will add to the toxic load of aluminum during the first months of life, so parents concerned about this should consider the ACTHIB version that does not contain aluminum. Receipt of HIB vaccines is also correlated with increased incidence of developing Type 1 Diabetes. It is unclear if the correlation is specific to this vaccine or a result of adding yet one more vaccine to the schedule and therefor increasing the amount of immune activation children are being subjected to.
Other Considerations: It should also be considered that use of HiB vaccines, the incidence of meningitis caused by Haemophilus Influenzae A and other strains has increased. Due to the increased incidence of Haemophilus strains not normally circulating, adults with no prior immunity to these newer strains are contracting these infections at higher rates.
The Vaccines: HiB vaccines are made by isolating sugars from the membrane of the bacteria. Since these sugars alone are not enough to make the body mount a sufficient immune response, they are either bound to the very reactive tetanus toxoid or injected along with an aluminum adjuvant.
Age Given: 2 months, 4 months, 6 months, 15 months
Package Insert information can be found here. https://www.immunize.org/fda/#hib
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
Vaccine Ingredients: ActHIB:
- HIG sugar/ tetaus toxoid complex
- sugar water
- saline solution
Vaccine Ingredients: PedVaxHIB:
- HIB sugar/ Neisseria protein complex
- saline solution
- Aluminum 225 micrograms
Pc: Pneumococcal Disease
The Illness: The bacteria Streptococcus pneumoniae causes an infection. It is transmitted through contact with infected person’s cough, mucus, or saliva. Infection is diagnosed with a blood test or spinal tap when a blood stream infection or meningitis is suspected. It is a common bacterial cause of respiratory infections. Treatment is IV antibiotics or oral antibiotics. The vaccine first became available in 2001.
Cost/Benefit Analysis: Infant bacterial infections can be serious and rarely, life-threatening. Infection with Streptococcus pneumoniae is the most likely cause of infant bacterial infection. Pneumococcal vaccines for infants do contain 125 micrograms of aluminum that will add to the toxic load of aluminum during the first months of life. Parents concerned about this should consider only vaccinating with one aluminum containing vaccine at a time. While vaccination with this vaccine can potentially reduce the risk of infant bacterial infection, it is important to understand how the infant immune system is designed and why. The reason that this vaccine includes the diphtheria toxin as well as aluminum is because the infant immune system is naturally under-reactive to the presence of bacteria (or its sugars in the case of the vaccine). Thus, the present of bacteria often does not induce an inflammatory response in infants. It is possible that the infant immune systems is designed this way in order to keep the body in an anti-inflammatory state during critical times of brain development, as high levels of inflammation can negatively affect brain development. To compensate for the under-responsiveness of the infant immune system to bacteria, breastmilk contains many antimicrobial components as well as antibodies against bacteria that are circulating in the environment. Thus, breastfed children have much lower incidence of bacterial infection than their bottle-fed counterparts.
Other Considerations: It is also important to note that the strains of this type of bacteria that are circulating in the environment are constantly changing, thus the vaccine is constantly being updated with new strains of bacteria. Thus, it went from PCV 7 to Prevnar 13 to Prevnar 20, with the number indicating the number of strains the vaccine is made against. Use of the vaccine induces strain replacement, whereby less prevalent, sometimes more deadly strains of the bacteria become more prevalent in the population. Thus, the vaccine must be continually updated to include the new variants. Similar to what is seen with HiB, the increased prevalence of the newer strains of these bacteria in the population in causing increases in severe bacterial infections in the elderly who do not have immunity to these once rare or newer strains.
The Vaccines: Pneumococcal vaccines are made by isolating sugars from the membrane of the bacteria. Since these sugars alone are not enough to make the body mount a sufficient immune response, they are bound to the very reactive diphtheria toxoid AND injected along with an aluminum adjuvant. This vaccine conjugates the bacterial sugars to a portion of the diphtheria protein called CRM197. This protein binds to receptors on cells and is internalized. This is of concern because there is the possibility that the immune system will attack cells which have internalized the CRM197 protein because it is foreign. CRM197 has been shown to increase the permeability of the blood brain barrier as well as to damage the cytoskeleton of cells, resulting in a loss of cell-to-cell contact. While there are several forms of this vaccine, the major difference is that they each cover a different number of strains of the Pneumococcal bacteria. Prevnar 13 and 20, and Vaxneuvance (PCV15) all use the CRM197 diphtheria protein. Prevnar 23 is NOT a conjugate vaccine, it merely has the membrane glycoproteins from each strain. Because this formulation is not enough to stimulate a strong immune response in infants, it is not recommended by the CDC for use in infants, but in adults over 50 years and children above 2 years who may need an additional booster following their standard pneumococcal vaccines.
Age Given: 2 months, 4 months, 6 months, 15 months
Package Insert information can be found here. https://www.fda.gov/media/149987/download?attachment
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
Vaccine Ingredients: While they differ slightly, all infant vaccines contain:
- Pc sugar/ diphtheria toxoid complex (CRM197 protein)
- Saline
- polysorbate 20 or 80
- Aluminum 125 micrograms
The Illness: Meningitis is a severe bacterial infection that can invade the spinal cord and brain and lead to death in a matter of days. The illness progresses from flu-like symptoms to high fever, severe headache and neck stiffness and vomiting often within 24 hours. It often is accompanied by a characteristic rash of red pinpoint dots that develop into larger purple patches over time. This bacterial infection quickly leads to systemic inflammation, known as sepsis, which can be fatal. The group most affected by this illness is college-aged persons, which comprise roughly 21% of cases. Fortunately, rates of meningitis in the U.S are at an all-time low. I have listed CDC surveillance data detailing the incidence of meningitis in various age groups to help you get an idea about the risk of infection.
Incidence in general population = 1 in 1.6 million persons
Incidence in infants 0-4 years = 1 in 300,000 persons
Incidence in children 5-14 years = 1 in 2.2 million persons
Incidence in persons 15-24 years = 1 in 670,000 persons
See the 2021 Enhanced Meningococcal Disease Surveillance Report
Again, although meningitis is rare, 1 in 5 cases can be fatal. Of survivors, about 15% end up with disabilities, including nerve damage, hearing loss, and limb amputation. For comparison, severe adverse events from vaccination with meningitis vaccines mostly range between 1-3% or 1-3 serious adverse events per 100 doses. Information concerning adverse events can be found in section 6 of each vaccine insert (linked below), which contains information on the clinical trials as well as the post-marketing data. Receiving multiple vaccinations at one appears to increase the rate to of adverse reactions for several meningitis vaccines.
Cost/ Benefit Analysis: Bacterial meningitis is extremely rare, yet extremely serious. The seriousness of this illness makes it an excellent candidate for a vaccine. Unfortunately, even though there are several vaccines against the strains of meningitis that have historically affected college-age adults, no evidence conclusively shows that they are effective at preventing this illness. The immunity provided by subunit and conjugate vaccines is known to wane quickly, often within months. Their efficacy is not measured by the real-world protection from meningitis but by the antibody response of the patient immediately following vaccination, making it difficult to assess their true efficacy. Vaccines against Neisseria meningitidis caused meningitis have been historically given to college-age persons, but are now being approved (but not yet “recommended”) for infants as young as 9 months. The lack of a recommendation for these vaccines in infants may be due to the fact that, historically, vaccines against bacteria have not stimulated a good immune response in infants. In college-aged persons, it appears that a significant percentage (>20%) of meningitis is from strains of the meningitis bacteria that are either unknown or not covered by current vaccines on the market.
The Vaccines: There are many strains of Neisseria meningitidis bacteria that can cause meningitis illness, the vaccines are made to cover specific strains, which include serogroups A, C, Y, W and B. The vaccine descriptions below list which serogroups are targeted by each vaccine. For most of the vaccines detailed below, sugars from the cell membrane of the meningitis bacteria are isolated and bound to a portion of the tetanus toxin to ensure that they stimulate a strong immune response. BEXSERO is an exception that uses meningitis bacterial proteins generated by recombinant DNA techniques (and then purified) to generate an immune response. Special caution should be taken with the MENVEO vaccine as it conjugates the bacterial sugars to a portion of the diphtheria protein called CRM197. This protein binds to receptors on cells and is internalized. This is of concern because there is the possibility that the immune system will attack cells which have internalized the CRM197 protein because it is foreign. CRM197 has been shown to increase the permeability of the blood brain barrier as well as to damage the cytoskeleton of cells, resulting in a loss of cell-to-cell contact.
Age Given: The CDC recommends vaccines against the A, C, Y, W serogroups of bacteria for children ages 11-12 yrs. and 16 yrs. However, many meningitis vaccines are now being approved for infants as young as 9 months.
Package Insert information can be found here. FDA Product Approval: View All (immunize.org)
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
Vaccine ingredients:
Menactra– against N. meningitidis serogroup A, C,Y, and W-135
Approved for persons 9 months through 55 years of age
- 4 mcg 3 each of meningococcal A, C, Y and W-135 polysaccharides conjugated to approximately 48 mcg 4 of diphtheria toxoid protein carrier
- sodium phosphate buffered isotonic sodium chloride solution
“In an analysis that took into account the missing data, estimates of the attributable risk of GBS 14 ranged from 0 to 5 additional cases of GBS per 1,000,000 vaccinees within the 6-week period 15 following vaccination.”
GBS stands for guillain-barre syndrome, a disorder where the immune system attack nerves, causing weakness and paralysis.
MENVEO- against N. meningitidis serogroup A, C, Y, and W-135
Approved for persons 9 months through 55 years of age
- 10 mcg MenA oligosaccharide; 5 mcg of each of MenC, MenY, and MenW-135 oligosaccharides; and 25.4 to 65.5 mcg CRM197 protein
- < 3 micrograms/mL formaldehyde
“Syncope (fainting) has occurred in association with administration of MENVEO. Procedures should be in place to avoid injury from fainting. (5.2) • Apnea following intramuscular vaccination has been observed in some infants born prematurely. A decision about when to administer MENVEO to an infant born prematurely should be based on consideration of the individual infant’s medical status and the potential benefits and possible risks of vaccination. (5.5)”
“Bell’s palsy showed a statistically significant increased risk in the period 1 to 84 days post vaccination compared with the control period, with an overall adjusted relative incidence of 2.9 (95% CI: 1.1-7.5). Among the 8 reported cases of Bell’s palsy, 6 cases occurred in persons who received MENVEO concomitantly with one or more of the following vaccines: Tdap, HPV, and Influenza vaccine.”
PENBRAYA– against N. meningitidis serogroup A, B, C, W, and Y
Approved for persons 10 years through 25 years old
- N. meningitidis serogroup A, C, W, and Y polysaccharide (5 mcg each; 20 mcg total) conjugated to tetanus toxoid (44 mcg tetanus toxoid)
- 2 recombinant lipidated factor H binding protein variants from N. meningitidis serogroup B (60 mcg each; total of 120 mcg protein)
- 0.78 mg histidine
- 0.097 mg trometamol
- 28 mg sucrose
- 0.25 mg aluminum phosphate
- 4.65 mg sodium chloride (4.65 mg)
- PS80 80 (0.018 mg) at pH 6.0
(these conjugate vaccines activated using chemicals such as 1-cyano-4(dimethylamino)-pyridinium tetrafluorobate (CDAP) and derivatized with adipic acid dihydrazide (ADH) and purified with ammonium sulfate, so residual amounts of these chemicals may remain.)
MenQuadfi- against N. meningitidis serogroup A, C, W, and Y
Approved for persons 2 years and older.
- 10 microgram each of meningococcal A, C, W, and Y polysaccharide antigens conjugated to approximately 55 micrograms tetanus toxoid protein carrier
- Saline
- 1.23 mg sodium acetate (30 mM)
- < 3 micrograms/mL formaldehyde
(these conjugate vaccines are produced and bound to toxin tetanus toxoid using chemicals such as carbonyldiimidazole (CDI), derivatized with adipic acid dihydrazide (ADH) and purified with ammonium sulfate, so residual amounts of these chemicals may remain.)
BEXSERO– against N. meningitidis serogroup B
Approved for persons 10-25 years of age
- 50 micrograms each of 3 recombinant proteins from N. meningitidis (grown in E. coli)
- 25 micrograms of bacterial cell membrane
- 1,500 micrograms aluminum hydroxide
- Saline solution
- 0.776 mg histidine
- 10 mg sucrose
- 0.01 micrograms kanamycin
- Serious adverse event rate of 2.0%

References
HiB: Haemophilus Influenzae Type B
- Bruce, Michael G et al. “Haemophilus influenzae serotype a invasive disease, Alaska, USA, 1983-2011.” Emerging infectious diseases vol. 19,6 (2013): 932-7. doi:10.3201/eid1906.121805 https://pubmed.ncbi.nlm.nih.gov/23735653/
- Adam, H J et al. “Changing epidemiology of invasive Haemophilus influenzae in Ontario, Canada: evidence for herd effects and strain replacement due to Hib vaccination.” Vaccine vol. 28,24 (2010): 4073-8. doi:10.1016/j.vaccine.2010.03.075 https://pubmed.ncbi.nlm.nih.gov/20398617/
- Sadeghi-Aval, Pouya et al. “Emergence of non-serotype b encapsulated Haemophilus influenzae as a cause of pediatric meningitis in northwestern Ontario.” The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale vol. 24,1 (2013): 13-6. doi:10.1155/2013/828730 https://pubmed.ncbi.nlm.nih.gov/24421786/
- Rubach, Matthew P et al. “Increasing incidence of invasive Haemophilus influenzae disease in adults, Utah, USA.” Emerging infectious diseases vol. 17,9 (2011): 1645-50. doi:10.3201/eid1709.101991 https://pubmed.ncbi.nlm.nih.gov/21888789/
- Resman, F et al. “Invasive disease caused by Haemophilus influenzae in Sweden 1997-2009; evidence of increasing incidence and clinical burden of non-type b strains.” Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases vol. 17,11 (2011): 1638-45. doi:10.1111/j.1469-0691.2010.03417.x https://pubmed.ncbi.nlm.nih.gov/21054663/
- Classen, John Barthelow, and David C Classen. “Clustering of cases of insulin dependent diabetes (IDDM) occurring three years after hemophilus influenza B (HiB) immunization support causal relationship between immunization and IDDM.” Autoimmunity vol. 35,4 (2002): 247-53. doi:10.1080/08916930290028175 https://pubmed.ncbi.nlm.nih.gov/12482192/
- Wahlberg, J et al. “Vaccinations may induce diabetes-related autoantibodies in one-year-old children.” Annals of the New York Academy of Sciences vol. 1005 (2003): 404-8. doi:10.1196/annals.1288.068 https://pubmed.ncbi.nlm.nih.gov/14679101/
- Classen, J B, and D C Classen. “Vaccines and the risk of insulin-dependent diabetes (IDDM): potential mechanism of action.” Medical hypotheses vol. 57,5 (2001): 532-8. doi:10.1054/mehy.2001.1352 https://pubmed.ncbi.nlm.nih.gov/11735306/
Pc: Pneumococcal Disease
- Mehtälä, Juha et al. “Competition between Streptococcus pneumoniae strains: implications for vaccine-induced replacement in colonization and disease.” Epidemiology (Cambridge, Mass.) vol. 24,4 (2013): 522-9. doi:10.1097/EDE.0b013e318294be89 https://pubmed.ncbi.nlm.nih.gov/23676265/
- Norton, Nancy B et al. “Routine pneumococcal vaccination of children provokes new patterns of serotypes causing invasive pneumococcal disease in adults and children.” The American journal of the medical sciences vol. 345,2 (2013): 112-20. doi:10.1097/MAJ.0b013e3182517785 https://pubmed.ncbi.nlm.nih.gov/22814362/
- Huang, Susan S et al. “Continued impact of pneumococcal conjugate vaccine on carriage in young children.” Pediatrics vol. 124,1 (2009): e1-11. doi:10.1542/peds.2008-3099 https://pubmed.ncbi.nlm.nih.gov/19564254/
- Dagan, Ron. “Serotype replacement in perspective.” Vaccine vol. 27 Suppl 3 (2009): C22-4. doi:10.1016/j.vaccine.2009.06.004 https://pubmed.ncbi.nlm.nih.gov/19545935/
Meningococcal Vaccine
- Mehtälä, Juha et al. “Competition between Streptococcus pneumoniae strains: implications for vaccine-induced replacement in colonization and disease.” Epidemiology (Cambridge, Mass.) 24,4 (2013): 522-9. doi:10.1097/EDE.0b013e318294be89 https://pubmed.ncbi.nlm.nih.gov/23676265/
- Norton, Nancy B et al. “Routine pneumococcal vaccination of children provokes new patterns of serotypes causing invasive pneumococcal disease in adults and children.” The American journal of the medical sciences 345,2 (2013): 112-20. doi:10.1097/MAJ.0b013e3182517785 https://pubmed.ncbi.nlm.nih.gov/22814362/
- Huang, Susan S et al. “Continued impact of pneumococcal conjugate vaccine on carriage in young children.” Pediatrics 124,1 (2009): e1-11. doi:10.1542/peds.2008-3099 https://pubmed.ncbi.nlm.nih.gov/19564254/
- Dagan, Ron. “Serotype replacement in perspective.” Vaccine 27 Suppl 3 (2009): C22-4. doi:10.1016/j.vaccine.2009.06.004 https://pubmed.ncbi.nlm.nih.gov/19545935/CDC Surveillance Data: https://www.cdc.gov/meningococcal/surveillance/surveillance-data.html
- Shinefield, Henry R. “Overview of the development and current use of CRM(197) conjugate vaccines for pediatric use.” Vaccine 28,27 (2010): 4335-9. doi:10.1016/j.vaccine.2010.04.072 https://pubmed.ncbi.nlm.nih.gov/20452430/
- Wang, Ping et al. “CRM197-induced blood-brain barrier permeability increase is mediated by upregulation of caveolin-1 protein.” Journal of molecular neuroscience : MN 43,3 (2011): 485-92. doi:10.1007/s12031-010-9471-5 https://pubmed.ncbi.nlm.nih.gov/21080104/
- Özerman Edis, Bilge et al. “Cross-reacting material 197 (CRM197) affects actin cytoskeleton of endothelial cells.” General physiology and biophysics 36,4 (2017): 383-389. doi:10.4149/gpb_2017006 https://pubmed.ncbi.nlm.nih.gov/28653650/
I am indebted to Dr. Robert Sears and Dr. Neil Miller for a substantial amount of the information concerning individual vaccines. For more information on each illness and vaccine, please see The Vaccine Book by Dr. Sears. For summaries of research studies concerning vaccines, please see Miller’s Review of Critical Vaccines Studies. Both books are indispensable references that belong on the bookshelf of every parent and vaccine safety advocate
Want to learn more about how you can recover your health?
Become a VI-TA member
(It's about the same as the price of a cup of Starbucks coffee!)
Toxoid Vaccines: DTaP, TDaP
The toxoid vaccines are designed to neutralize the toxins produced during infection with the diphtheria, tetanus or pertussis bacteria. Neutralizing toxins alleviates the symptoms of these illnesses.
Toxoid vaccines do NOT prevent the spread of these bacterial illnesses, they only alleviate symptoms while immunity persists.
How Are They Made?
The symptoms of these infections are caused by bacterial toxins. The bacterial toxins are isolated, purified and used in the vaccine. They prime the immune system to neutralize the toxin should the body see it again, thereby preventing symptoms. This vaccine does not prevent infection by or spread of the bacteria.
Pros
- Able to mitigate life-threatening symptoms of whooping cough (Pertussis) in infants
Cons
- Recipients become asymptomatic carriers when exposed to Pertussis bacteria.
- Pertussis immunity is very short-lived.
- New strains of bacteria are evolving that are not covered by the vaccine.
- Fail to activate cellular immunity.
- Contains aluminum, which is a known neurotoxin.
Often contain aluminum, which is a known neurotoxin.
DTaP
The Illnesses: The DTaP vaccine is actually 3 vaccines combined that are designed to prevent symptoms (but not contracting) of 3 different illnesses.
Diphtheria is a bacterial infection with the Corynebacterium diphtheriae bacterium. During the infection, a toxin secreted by the bacteria irritates the throat and upper lungs, causing coughing and breathing difficulty. It is transmitted like the common cold and diagnosed with throat swap and lab test. Diphtheria is almost unheard of in the U.S, with a maximum of 5 cases a year in the US. However, if contracted, it can have as high as a 10% fatality rate. Antitoxin is given as treatment as well as intravenous antibiotics. The pathogenesis of this infection is now better understood. The bacteria Corynebacterium diphtheriae only cause Diphtheria when they are infected with a virus that infects bacteria, called a prophage.1 The prophage infected bacteria produce the diphtheria toxin only when they are in a low iron environment.1 Conversely, a high-iron environment leads to repression of toxin production.1
The bacteria that causes Tetanus (also known as lockjaw) does so by producing a toxin. Thus, it is the presence of the tetanus toxin that causes paralysis. Tetanus usually results from the Tetanus bacteria entering a deep wound where it can grow without oxygen present (needs an anaerobic (without oxygen) environment. Tetanus bacteria are found in animal feces and is common in farm environments. Cases of Tetanus are rare, with about 50 to 100 cases occurring per year in the US. About 1 child under 5 yrs old per year is diagnosed with active Tetanus in the U.S. Antibody injections and antibiotics can help get rid of the bacteria, but there is no antitoxin. Intensive care/ life support is needed while it runs it’s course. However, data from a study linked to below suggest the possibility that Vitamin C (ascorbic acid) may be able to mitigate the deleterious effects of the tetanus toxin.2
Pertussis or Whooping Cough is caused by infection with the bacteria Bordetella pertussis. During infection, the pertussis toxin secreted by the bacteria irritates the throat and upper lungs, causing coughing and breathing difficulty. Pertussis can be life-threatening for infants under 6 months old and very serious for older infants. In teens and adults the symptoms often mimic those of bronchitis. It is transmitted like the common cold and can last for up to three months. Pertussis infection is diagnosed by the distinctive cough or by nasal swab and lab test. Pertussis infection is extremely common, with about 10,000 cases reported each year in the US during the 1990s and 2000s. It has been making a resurgence since the advent of the newer acellular Pertussis vaccine, likely because the newer vaccine does not prevent transmission of the Pertussis bacteria, but works by neutralizing the pertussis toxin.4 Also, Bordetella parapertussis causes similar symptoms, but no toxin.7 There is no vaccine for B. parapertussis.
Treatment is antibiotics. If coughing interferes with breathing oxygen is needed. There are some reports by physicians that treatment with high-dose vitamin C (ascorbate) can lessen the acute symptoms of Pertussis.
The rates of Tetanus infection and Diphtheria infection in the U.S are so low that the risk of a vaccine adverse reaction outweighs the risk of harm from either illness. However, severe illness from Pertussis infection is quite common, comprising some of the 10,000 to 20,000 cases/year. The risk of death from Pertussis for infected infants under 6 months old is about 1%, or 20 deaths per year. However, infants and children who recover have no long-term side effects following infection (other than lifelong immunity). The mechanism by which all three vaccines protect is by directing the body to make antibodies that neutralize the toxin produced by each type of bacteria. Thus, following vaccination against Pertussis, the body makes antibodies that neutralize pertussis toxin. Since it is the toxin that causes severe pulmonary symptoms, such as the “whooping” cough, neutralization of the toxin prevents severe illness. What vaccination does NOT do in each of these cases is prevent persons from getting infected with the bacteria.4, 5, 6 Thus, vaccination does NOT prevent transmission of these illnesses (while recovery from infection does give immunity that protects from re-infection and transmission to others).4, 5, 6 This presents a significant risk in families with newborn infants. Toddler siblings of newborns are likely to contract the pertussis bacteria in daycare settings. These toddlers are usually vaccinated, and are protected from the symptoms of the illness, so they become asymptomatic carriers of the pertussis bacteria. They can then spread the pertussis bacteria to their unvaccinated newborn brother or sister, which can lead to severe illness. Public Health advocates have tried to avert this problem by encouraging vaccination with DTaP during pregnancy. However, immune activation in the mother and fetus during this critical time of brain development for the infant is correlated with higher risk of neurological dysfunction and autism.3
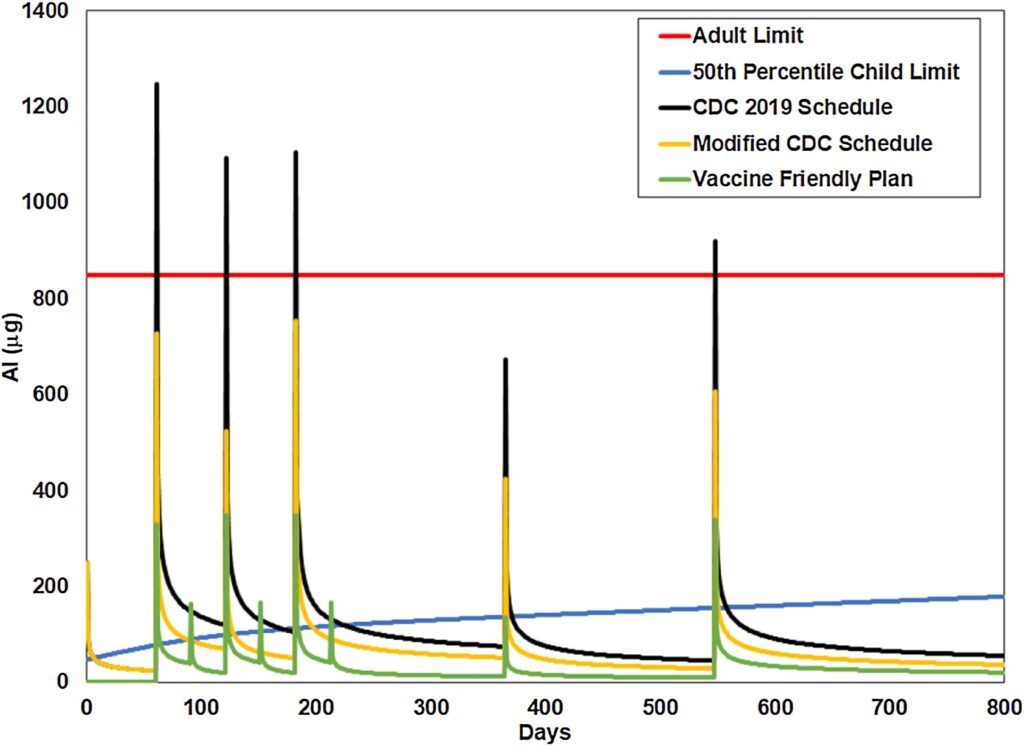
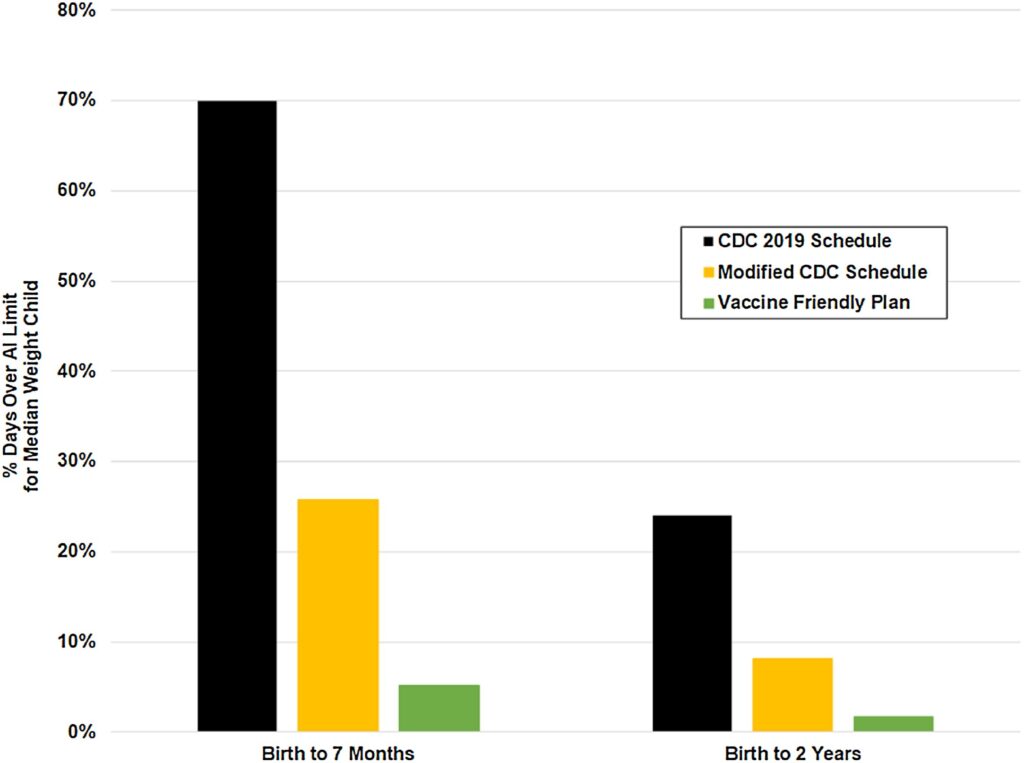
All brands of DTaP currently contain aluminum, although at amounts that differ widely from one another. Thus, parents may want to choose the brand with the lowest amount of aluminum. The presence of aluminum DTaP is concerning because it is given at 2, 4 and 6 months, greatly contributing to the overall aluminum load in the infant as well as creating at state of chronic immune activation can impair proper brain development and contributes to the development of neurological dysfunctions.3 A recent study demonstrated that children vaccinated according to CDC guidelines were in a state of aluminum toxicity more than 70 % of their first 7 months of life.10 Infants vaccinated according to an alternative vaccine (developed by Dr. Paul Thomas, MD) schedule that reduced the number and increased the spacing of aluminum containing vaccines reduced the amount of time in aluminum toxicity to 5%.10
Previous formulations of DTaP vaccines contained a form or mercury called thimerosal (that is used as a preservative). These versions were significantly more likely to be associated with the diagnosis of autism spectrum disorders.12
All three vaccines were developed with the goal of neutralizing the bacterial toxin that causes the life-threatening symptoms of the disease. Thus, a toxoid (partial toxin) for each disease is injected into the body, along with an aluminum adjuvant to stimulate the body to produce antibodies against the toxin/toxoid. Since the goal is to neutralize the destructive toxin, none of the vaccines were designed to prevent the spread of the bacteria that cause infection. Thus, mass vaccination creates a population of persons who become asymptomatic spreaders of the harmful bacteria. Chronic use of vaccines that do NOT stop transmission also result in selection pressure on the bacteria, which has led to the proliferation of many different strains of pertussis that the vaccines offer no protection from.6, 7 Concerningly, receipt of the Dtap vaccine by teenagers is associated with 20 times increased risk of developing thrombocytopenia (a severe bleeding disorder).15
Severe allergic reaction (anaphylaxis) or encephalopathy following a previous vaccination. Progressive neurological disorder, such as infantile spasms, uncontrolled epilepsy, or progressive encephalopathy.
Development of Gullian-Barre Syndrome has been causally related to receipt of DTaP vaccines. DTaP vaccination may result in apnea (stop breathing) when given to infants born prematurely.16, 17 In post-marketing studies, receipt of DTaP was associated with allergy13, anaphylaxis, blood and lymph disorders, cellulitis, convulsions, seizures14, fainting, and screaming.
2, 4, 6 months, 18 months, 5 years, 12 years (Tdap).
Package Insert information can be found here. https://www.immunize.org/fda/#dtap
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
(General, may vary by manufacturer)
- Three germ and toxoid components
- Saline Solution
- 2-phenoxyethanol
- Polysorbate 80 100 micrograms
- Formaldehyde 100 micrograms
- Aluminum- amount varies by vaccine manufacturer
- Daptacel (Sanofi Pasteur)- 330 micrograms aluminum
- Tripedia (Sanofi Pastuer)- 170 micrograms aluminum
- Infantrix (GlaxoSmithKline)- 625 micrograms aluminum
References
- Holmes, R K. “Biology and molecular epidemiology of diphtheria toxin and the tox gene.” The Journal of infectious diseases 181 Suppl 1 (2000): S156-67. doi:10.1086/315554 https://pubmed.ncbi.nlm.nih.gov/10657208/
- Jahan, K et al. “Effect of ascorbic acid in the treatment of tetanus.” Bangladesh Medical Research Council bulletin 10,1 (1984): 24-8. https://pubmed.ncbi.nlm.nih.gov/6466264/
- Autism & Aluminum Adjuvants in Vaccines: How Aluminum Adjuvants in Vaccines can Cause Autism. http://vaccinepapers.org/wp-content/uploads/Autism-and-aluminum-adjuvants-in-vaccines-1.pdf
- Gill, Christopher et al. “The relationship between mucosal immunity, nasopharyngeal carriage, asymptomatic transmission and the resurgence of Bordetella pertussis.” F1000Research 6 1568. 25 Aug. 2017, doi:10.12688/f1000research.11654.1 https://pubmed.ncbi.nlm.nih.gov/28928960/
- Warfel JM, Zimmerman LI et al. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci 2014 Jan 14; 111(2): 787-92. https://pubmed.ncbi.nlm.nih.gov/24277828/
- Mooi FR, Van Der Maas NA, De Mellker HE. Pertussis resurgence: waning immunity and pathogen adaptation—two sides of the same coin. Epidemiol Infect 2014 Apr; 142(4): 685-94. https://pubmed.ncbi.nlm.nih.gov/23406868/
- Hegerle N, Paris AS, et al. Evolution of French Bordetella pertussis and Bordetella parapertussis isolates: increase of Bordetellae not expressing pertactin. Clin Microbiol Infect 2012 Sep; 18(9): E340-6. https://www.sciencedirect.com/science/article/pii/S1198743X14610508
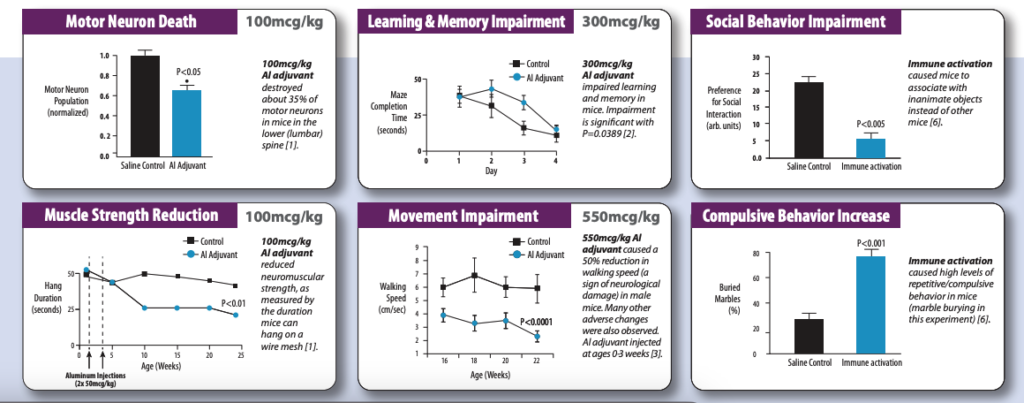
- Shaw, Christopher A, and Michael S Petrik. “Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration.” Journal of inorganic biochemistry 103,11 (2009): 1555-62. doi:10.1016/j.jinorgbio.2009.05.019 https://pubmed.ncbi.nlm.nih.gov/19740540/
- Shaw, C A et al. “Administration of aluminium to neonatal mice in vaccine-relevant amounts is associated with adverse long term neurological outcomes.” Journal of inorganic biochemistry 128 (2013): 237-44. doi:10.1016/j.jinorgbio.2013.07.022 https://pubmed.ncbi.nlm.nih.gov/23932735/
- McFarland, G., La Joie, E., Thomas, P, Lyons-Weiler (2019). Acute exposure and chronic retention of aluminum in three vaccine schedules and effects of genetic and environmental variation. Journal of Trace Elements in Medicine and Biology, March 2020. https://pubmed.ncbi.nlm.nih.gov/31846784/
- Thomas, P., & Margulis, J. (2016). The vaccine-friendly plan: Dr. Paul’s safe and effective approach to immunity and health — from pregnancy through your child’s teen years. New York, NY: Ballantine Books
- Geier, David A. et al. “The risk of neurodevelopmental disorders following a Thimerosal-preserved DTaP formulation in comparison to its Thimerosal- reduced formulation in the vaccine adverse event reporting system (VAERS).” (2014). https://www.semanticscholar.org/paper/The-risk-of-neurodevelopmental-disorders-following-Geier-Kern/9c310147e02a126fc582ef51f226bb82c4848f4d
- McDonald, Kara L et al. “Delay in diphtheria, pertussis, tetanus vaccination is associated with a reduced risk of childhood asthma.” The Journal of allergy and clinical immunology 121,3 (2008): 626-31. doi:10.1016/j.jaci.2007.11.034 https://pubmed.ncbi.nlm.nih.gov/18207561/
- Barlow, W E et al. “The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine.” The New England journal of medicine 345,9 (2001): 656-61. doi:10.1056/NEJMoa003077 https://pubmed.ncbi.nlm.nih.gov/11547719/
- O’Leary, Sean T et al. “The risk of immune thrombocytopenic purpura after vaccination in children and adolescents.” Pediatrics 129,2 (2012): 248-55. doi:10.1542/peds.2011-1111 https://pubmed.ncbi.nlm.nih.gov/22232308/
- Flatz-Jequier, Aline et al. “Recurrence of cardiorespiratory events following repeat DTaP-based combined immunization in very low birth weight premature infants.” The Journal of pediatrics 153,3 (2008): 429-31. doi:10.1016/j.jpeds.2008.03.043 https://pubmed.ncbi.nlm.nih.gov/18718262/
- Furck, A K et al. “Very low birth weight infants have only few adverse events after timely immunization.” Journal of perinatology : official journal of the California Perinatal Association 30,2 (2010): 118-21. doi:10.1038/jp.2009.112 https://pubmed.ncbi.nlm.nih.gov/19710678/
I am indebted to Dr. Robert Sears and Dr. Neil Miller for a substantial amount of the information concerning individual vaccines. For more information on each illness and vaccine, please see The Vaccine Book by Dr. Sears. For summaries of research studies concerning vaccines, please see Miller’s Review of Critical Vaccines Studies. Both books are indispensable references that belong on the bookshelf of every parent and vaccine safety advocate.
Want to learn more about Vaccine Injury treatments? Join the VI-TA membership.
(It's about the same as the price of a cup of Starbucks coffee!)
Inactivated Virus/Viral Antigen Vaccines
Inactivated viral vaccines come from viruses that are first grown on cells. They are then harvested and chemically inactivated in some way so that they cannot cause infection.
How Are They Made?
Live viruses are grown on animal cells and then harvested and purified. The purified viruses are inactivated with various chemicals. Inactivated virus vaccines often contain aluminum adjuvants to help them better stimulate an immune response. *A portion of the virus’ protein coat is made by using recombinant technology and is used to produce the vaccine.
Pros
- Cannot get illness from the vaccine
Cons
- Immunity is often short-lived.
- Fail to activate cellular immunity.
- Often contain aluminum, which is a known neurotoxin.
Often contain aluminum, which is a known neurotoxin.
The Illness: Poliovirus is a virus that infects the gut lining (enterovirus). Infection usually results in mild symptoms or is asymptomatic. However, rarely, in about 1% of cases, the poliovirus is able to move from the gut cells to cells within the nervous system and spinal cord, leading to infection of the nervous tissue.2 This results in weakness and paralysis. During Polio outbreaks in the 1950’s, cases of paralytic Polio peaked at 40 per 100,000 people.2 However, currently, there has not been a case of Polio recorded in the United States since 1985, when a traveler with Polio came to the U.S. It is transmitted through oral-fecal routes, typically through the ingestion of contaminated water as well as like the common cold.
Cost/Benefit: Risk of an adverse reaction greatly outweighs any benefit of this vaccine due to the fact that there is no Polio in U.S. Further, the vaccine against Polio that is currently used in the U.S. is an inactivated viral vaccine that has very low efficacy, as measured by seroconversion (the production of anti-Polio antibodies).7 If overseas travel is a consideration, it is difficult to assess the true effectiveness (and cost/benefit) of the vaccine in preventing Polio infection due to the fact that there is such low circulation of the wildtype Poliovirus. Thus, no studies actually measure the ability of this vaccine to prevent Polio infection.
The Vaccines: The current vaccine used against Polio in the United States is an inactivated viral vaccine. Three strains of live Polio virus are grown in monkey kidney cells that lack anti-viral defenses (VERO cells). The live viruses are isolated and inactivated using formalin. The growth of viruses in VERO cells is problematic because these cells have been shown historically to harbor a variety of monkey viruses and may also harbor other contaminating viruses.1 2,3,13,14 The presence of glutamate in the vaccine may trigger an allergic-type reaction in some people.
The first vaccines made against Polio were also grown in monkey kidney cells (VERO cells). 1,2 3 When the vaccine was injected into hamsters, the hamsters grew tumors. 1,2,3 Scientists set about isolating contaminating monkey viruses that were suspected to be the cause of the tumor growth and finally isolated Simian Virus 40 (SV40), which is now studied as a prototypical cancer-promoting virus. 1,2,3,8 At the time, the choice was made to go ahead and give millions of Americans SV40 contaminated Polio vaccines under the assumption that it would not cause problems. 1 2,3 SV40 has now routinely been isolated from many human tumors, and there is significant data implicating SV40 in the growth and metastasis of these tumors.3,8 Moreover, it is clear that viral infection with SV40 has been passed down generationally, causing it to be endemic in the human population.8 While current VERO cell lines may not be contaminated with the SV40 virus, it is possible that they harbor other contaminating viruses that may make it into the final vaccine preparation.9 Even though all viruses will have been made inactive, the possibility that there may be contaminating viral DNA or RNA in the vaccine is problematic. Medically, the presence of viral genetic material, especially retroviruses, can theoretically cause several problems. First, the genetic material may bind to Toll-like receptors and activate an aberrant immune response.10,11 Second, the residual DNA may be taken up by stem cells in the blood and insert itself into the host DNA, thereby causing insertional mutagenesis.11 Insertional mutagenesis will increase the risk of cancers, specifically blood cancers such as leukemia and lymphoma.11
While only inactivated Polio vaccine is used in the U.S., live viral vaccines containing 3 strains of Poliovirus are used overseas. When live viruses are used to vaccinate, there is always the possibility that the virus will mutate back to its more virulent form during infection.4,5 Unfortunately, this is exactly what has happened with Strain 2 of the live Polio vaccine. 4,5 As a result, vaccine strain Polio is now infecting many people worldwide and causing new epidemics of Polio and increased incidence of paralytic Polio. So, while wild Polio is relatively rare worldwide, vaccine-strain Polio is becoming more and more common and problematic. 4,5 Another problem associated with the use of live oral Polio vaccines overseas is that increases in acute flaccid paralysis (clinically indistinguishable from Polio) are seen following Polio vaccination campaigns.6 This suggests that these vaccination campaigns may be resulting in more paralysis than they are preventing.6
Age Given: 2, 4 months, 18 months, 5 years
Package Insert information can be found here. https://www.immunize.org/fda/#ipv
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
Vaccine Ingredients:
- Ipol (Sanofi Pasteur)
- 3 inactivated virus strains
- M-199 culture medium (saline, vitamins, amino acids, sucrose, glutamate, and human albumin)
- 2-phenoxyethanol
- formaldehyde
- residual amounts of three antibiotics
- traces of calf serum
More information on the historical contamination of Polio vaccines with Simian Virus 40 (SV40) can be found in the following articles/books.
- “The Virus and the Vaccine” article by printed in The Atlantic
- “The Virus and the Vaccine” book by Debbie Bookchin & Jim Schumacher
- “Dissolving Illusions” book by Suzanne Humphries
- “The Moth in the Iron Lung” book by Forrest Maready
More information on the potential contamination of Polio vaccines and other vaccines with retroviruses can be found in the following articles/books.
- “Plague” book by Judy Mikovits (re-pleat with scientific details)
- “Plague of Corruption” book by Judy Mikovits (easier read for the layperson)
The Illness: Hepatitis A is a virus that attacks the liver and causes temporary liver inflammation. Most children who get the virus don’t have any symptoms at all. Teens and adults who get sick usually experience intestinal flu symptoms that can last for a few weeks. Often jaundice will occur in teens and adults and that’s when the illness is usually diagnosed, with a blood test.
Hepatitis A is generally transmitted through the stool, through lack of proper hygiene. It can also be transmitted through blood transfusion or needle sharing.
Cost/Benefit: Hepatitis A is generally very mild in children, as most are largely asymptomatic. Infection then confers lifelong immunity that will protect them from possibly getting a more severe Hepatitis A infection when they are older. Inactivated viral vaccines such as this one usually give longer lasting immunity than subunit vaccines and may not mis-train the immune system in the same deleterious ways that sub-unit vaccines do. However, given some of the serious concerns detailed below, this vaccine is mostly risk with little to no benefit. Thus, I see no need to even consider this vaccine until a child is a preteen if they do not have immunity as indicated by positive antibody titers. The vaccine contains 250 micrograms of aluminum, which can contribute to chronic immune activation and other toxicities. The virus used to make the vaccine is inactivated by the chemicals (formaldehyde) added to it. However, there have been cases historically, such as with the Polio vaccine, where the virus survived the chemical inactivation process. More concerningly, Hepatitis vaccines contain residual human cell debris and DNA/RNA from the cell line they were grown in. Both versions of the vaccine were grown in a cell line derived from an aborted male fetus (MCR5). This raises ethical concerns for many people. Medically, the presence of human cell debris can theoretically cause several problems. First, the cell debris and/or the genetic material may bind to Toll-like receptors and activate an aberrant immune response. Second, the residual DNA may be taken up by stem cells in the blood and insert into the host DNA, thereby causing insertional mutagenesis. Insertional mutagenesis will increase the risk of cancers, specifically blood cancers such as leukemia and lymphoma. Third, residual human cell debris is often contaminated with human retroviruses, such as HERV-K, which are associated with chronic fatigue and cancer.
The Vaccines: Both commonly given vaccines (Vaqta by Merck and Havrix by GSK) are inactivated viral vaccines. A weakened version of the Hepatitis A virus is grown on human cells derived for an aborted male fetus (MCR5 cells) to produce the virus. The virus is then harvested from the cells and inactivated with formaldehyde and/or other chemicals. Aluminum (250 micrograms) is added to help the vaccine induce a more robust immune response. As a result of being grown in human cells, the final vaccine product WILL contain residual human cell debris and DNA/RNA. In clinical trials for Havrix, unsolicited adverse events were recorded on the diary card for 31 days after vaccination. Telephone follow-up was conducted 6 months after the last vaccination to inquire about serious adverse events, new onset chronic illnesses, and medically significant events. In clinical trials for Vaqta, infection site reactions were monitored for 5 days after injection and systemic reactions were monitored for 15 days following injection. Both vaccines show a correlation with gastrointestinal problems and administration of the Havrix vaccine shows a correlation anorexia. Some studies also show a correlation between receipt of the Hepatitis A vaccine and the development of thrombocytopenia, a disorder that causes life-threatening internal bleeding. The Hepatitis A vaccine was not given routinely before 2006.
Age Given: 1 year, and 18 months
Package Insert information can be found here. https://www.immunize.org/fda/#hepa
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
Vaccine Ingredients: Vaqta (Merck)
- Hepatitis A virus
- Aluminum 250 micrograms
- saline solution
- residual proteins and DNA from the human cell line
- traces of cow blood proteins
- formaldehyde
- sodium borate
- “other residual chemicals at a level of 10 parts per billion”
The Illness: The Flu is a respiratory virus that is usually mild and rarely serious. Serious infection is most likely to affect the elderly, whose immune function is not optimal.
Cost/Benefit: There is very little benefit to the flu vaccine for several reasons. It design does NOT enable it to effectively prevent transmission of the influenza virus because it does not produce mucosal immunity the way infection with the live virus does. The Flu vaccine is largely ineffective because what immunity it does confer wanes quickly and the vaccine is rarely a good match to the strain in circulation. Multi-dose vials also still contain large amounts of the mercury-based preservative, thimerosal. This type of inactivated vaccine also mis-trains the immune system in such a way that the body only neutralizes flu strains that are almost an exact match to the vaccine, often causing the immune system to largely ignore new variants. This allows new variants to set up an strong infection. While flu vaccination is somewhat effective in the first year the influenza vaccine is given, studies have shown that vaccination against flu in multiple successive years results in greater incidence of hospitalizations for both flu and non-influenza respiratory infections in both children and adults. The book Vax-Unvax: Let the Science Speak, by Dr. Brian Hooker summarizes this information quite well using easy to read graphs. A study by Rikin et. al. showed that children 4 yrs. old and younger that were vaccinated against influenza were 4.8 times more likely to suffer from a respiratory infection other than the flu. Similarly, another study by Cowling et. al. demonstrated that vaccination did not really affect the incidence of influenza infection in children between the ages of 6 and 15 yrs. old. However, children vaccinated against influenza were 4.40 times more likely to suffer from a non-flu respiratory infection. Importantly, Joshi et. al. found that children vaccinated against influenza were 3.67 times more likely to be hospitalized for influenza respiratory infection.
The Vaccines: There are at least nine different brands of Flu vaccine. Some are licensed for children, while others are not. Most are grown on tissue culture derived from chicken embryos. Many manufacturers have taken the mercury-based preservative, thimerosal, out of their vaccine in the last few years. Unless explicitly noted to contain mercury, the vaccines listed below do NOT contain significant amounts of mercury (as per the package insert, which I have linked to).
Age Given: Once yearly from 6 months of age
Package Insert information can be found by clicking on the name of each brand of Flu vaccine.
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
Flu Vaccine Ingredients by Vaccine Manufacturer
Approved for infants 6 months and older
- Multidose vials contain MERCURY in the form of Thimerosal at a concentration of
- [24.5 micrograms MERCURY in the 0.5 ml dose]
- [12.25 micrograms MERCURY in the 0.25 mg dose]
- 4 strains of the Influenza virus
- Phosphate buffered saline
- Calcium chloride
- Sodium taurodeoxycholate (to inactive virus)
- Beta-propiolactone (to inactivate virus)
- sucrose
- ovalbumin
- hydrocortisone
- neomycin (antibiotic)
- polymyxin
- Multidose vials contain MERCURY in the form of Thimerosal at a concentration of
- [24.5 micrograms MERCURY in the 0.5 ml dose]
- [12.25 micrograms MERCURY in the 0.25 mg dose]
- Subunits from 4 strains of the Influenza virus
- grown in Cocker Spaniel kidney cells (MDCK cells)
- Beta-propiolactone (to inactivate virus)
- Cetyltrimethylammonium bromide (detergent)
- 4 strains of influenza virus (killed/split)
- Octoxynol (Triton X-100)
- Alpha-tocopheryl hydrogen succinate
- Polysorbate 80 (Tween 80)
- Sodium deoxycholate
- Gentamicin sulfate
- ovalbumin
- hydrocortisone
- residual egg protein
- 4 strains of influenza virus (killed/split)
- Alpha-tocopheryl hydrogen succinate
- Polysorbate 80 (Tween 80)
- Sodium deoxycholate
- formaldehyde
- ovalbumin
Multidose vials contain MERCURY in the form of Thimerosal at a concentration of
- [25 micrograms MERCURY in the 0.5 ml dose]
- 4 strains of influenza virus (killed/split)
- Octoxynol (Triton X-100)
- Phosphate buffered saline
- Formaldehyde
- residual egg protein
Approved for at least 18 years or older
Approved for 18 and older
- 4 flu strains grown in Sf9 cells of the fall armyworm (insect)
- Phosphate buffered saline
- Tween 20 (polysorbate 20)
Approved for 65 yrs and older
- Uses MF59 (a squalene-based oil-in-water emulsion) as an adjuvant
- Residual egg protein
- Fomaldehyde
- Single dose has neomycin, kanamycin, hydrocortisone
The Illness: The Hepatitis B virus can cause liver damage, sometimes liver failure, and if rare instances, can be fatal.
There is no routine treatment available during the acute phase of the disease. Fortunately, in most adults it passes without much consequence. For kids, it is more likely to cause long-term problems. If one continues to carry Hepatitis B as a chronic disease, treatment is available with a medication similar to chemotherapy. About 1/3 of people are cured by this treatment. Pregnant mothers can be tested for Hepatitis B and babies with an infected mother can be given hep B antibody injection called HBIG.
Hepatitis B is transmitted sexually or through dirty IV drug needles or very rarely through blood transfusion or through birth from an infected mother.
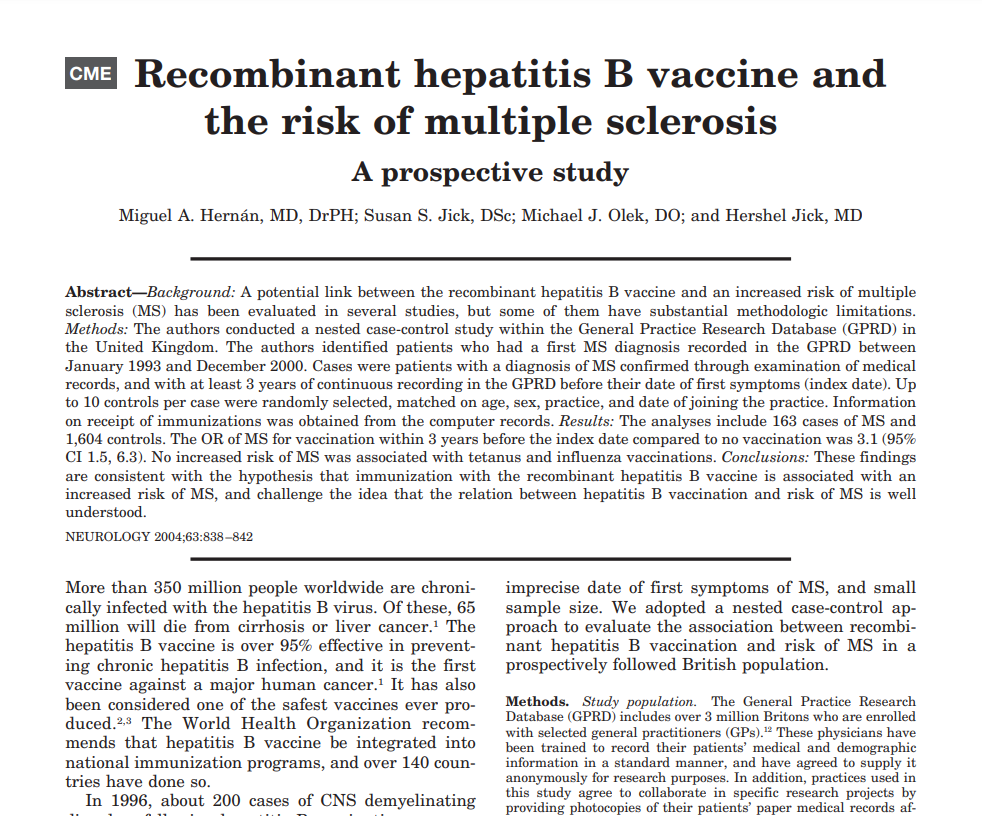
Cost/ Benefit Analysis: For children, the risk of contracting Hepatitis B is miniscule, since it is spread via blood or sexual intercourse. Thus, the risks of vaccination (detailed below) for most will far exceed the benefit. Adults should assess their own perceived risk for illness and balance against the potential risks of vaccination. Vaccines against hepatitis B are reported to cause more adverse reactions than other vaccines. Concerningly, relative to other vaccines, such as those against influenza or tetanus, receipt of Hepatitis B vaccine is strongly associated with the onset of autoimmune illness, most notably multiple sclerosis (see references below). It is highly unlikely that autoimmunity would be picked up in the clinical trials as an adverse event because the participants were only monitored for adverse events for either 4 or 5 days following vaccination (package insert, section 6). One of the most concerning aspects of the Hepatitis B vaccines is that they contain 250 micrograms of aluminum and are given on the day of birth. A typical infant can safely receive no more than 25 micrograms of aluminum per day, so the receipt of 10 times that amount on the day of birth is significant cause for concern. Hepatitis B vaccine is the first of multiple aluminum containing vaccines administered at birth, 2, 4 and 6 months. The chronic immune activation caused by serial receipt of aluminum containing vaccines is deleterious in many ways. Two key concerns are that 1) repeated vaccination with aluminum containing vaccines compromises innate immune response and 2) macrophages that take up aluminum have been shown to travel to the brain (in animal studies) where they create a state of chronic inflammation, as measured by interleukin-6 activation.
The Vaccines: Hepatitis B vaccines are subunit vaccines made using recombinant DNA techniques
Age Given: at birth, 1 or 2 months of age
Package Insert information can be found here. https://www.immunize.org/fda/#hepb
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
Vaccine ingredients: Recombivax HB (Merck)
- Hep B surface antigen
- Aluminum 250 micrograms
- Saline solution
- Yeast proteins
- Formaldehyde- residual amount
Vaccine ingredients: Engerix-B (GlaxoSmithKine)
- Hep B surface antigen
- Aluminum 250 micrograms
- Saline solution
- Yeast proteins
The Illness: The Human Papillomavirus and risk of cervical cancer
HPV is a virus that causes genital warts, which are spread through sexual contact. There are about 200 strains of the virus which differ in both their ability to cause warts and their ability to cause the cellular dysregulation that can lead to cervical cancer. Fortunately, the body is able to clear this viral infection with no adverse consequences in upwards of 90% of people. When HPV does not clear on its own, infected tissues only turn into cancer in 0.15% of the individuals who are unable to clear the infection over a timeframe of 10 years. Cervical cancer is a very slow-progressing cancer. Importantly, pap smears are a highly effective method of identifying lesions that could develop into cervical cancer and treatment is available for these lesions.
Cost/ Benefit Analysis: Risk for cervical cancer is very low in the United States. Cervical cancers only represent 0.7% of all new cancer cases. Approximately 2 in 100,000 women die of cervical cancer each year. As mentioned above, it is generally very slow growing and easily identified with a pap smear and easily treated when caught early. The effectiveness of the HPV vaccines was not assessed by measuring their impact on the development of cancer because that would have required years of follow-up. Instead, efficacy was measure by the ability of the vaccine to prevent the development of abnormal cells after exposure to HPV strains covered by the vaccine. Because the vaccines are only made to the most common strains of genital warts, the virus continues to mutate and produce new variants that may or may not be found to be associated with the development of cervical cancer. Safety of the Gardasil vaccine is a major concern, with a total of 67,550 adverse events recorded in VAERS as of November 25, 2002. Prior to the onset addition of COVID vaccines, an analysis of the VAERS database by Tomljenovic et. al. (2013) showed that HPV vaccination was associated with more than 60% of all life-threatening adverse reactions and 82% of all reported permanent disability in females under 30 years of age. As a matter of fact, so many legitimate adverse events were reported post vaccination with Gardasil that the American College of Pediatricians felt prompted in 2016 to issue a statement describing their concerns, particularly related to the risks of ovarian failure and autoimmune association with the adjuvant used in Gardasil and demanding more safety research be done. Merck, itself, acknowledges the vaccine-induced autoimmune risk in the product’s medical insert, stating that “2.2% of Gardasil 9 recipients reported new medical conditions potentially indicative of systemic autoimmune disorders”. Other safety signals include multiple sclerosis, ALS, paralysis, GBS, convulsions, chronic fatigue syndrome, autonomic dysregulation, damage to the nervous system, anaphylaxis, thrombocytopenia, vasculitis, menstrual issues, pulmonary embolism and death. As was noted above, many of the systemic reactions associated with the HPV vaccine may be related to the nanoparticulate amorphous aluminum adjuvant that is used in them to stimulate a strong immune response. Given that there is another method of preventing cervical cancer through non-invasive Pap screenings, and given the high rate of adverse events and the potential for post-vaccination ovarian failure and autoimmune disorders, the HPV vaccine is almost all risk with little to no benefit.
The Vaccines: HPV vaccine is a vaccine that uses protein subunits of 9 strains of the virus, which are made and obtained using recombinant DNA techniques.
Age Given: 3 doses between 9-14 years of age
Package Insert information can be found here. FDA Product Approval: View All (immunize.org)
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
Vaccine ingredients: Gardasil (Merck)
- Proteins from 9 strains of HPV
- Aluminum (as Amorphous Aluminum Hydroxyphosphate Sulfate or AAHS) – 500 micrograms
- Saline solution
- L-histidine (an amino acid)
- Yeast proteins
- Sodium Borate- 35 micrograms
- Polysorbate 80- 50 micrograms
References
Polio
- “The Virus and the Vaccine” book by Debbie Bookchin & Jim Schumacher
- “Dissolving Illusions” book by Suzanne Humphries
- Institute of Medicine (US) Immunization Safety Review Committee. Immunization Safety Review: SV40 Contamination of Polio Vaccine and Cancer. Edited by Kathleen Stratton et. al., National Academies Press (US), 2002. doi:10.17226/10534 https://pubmed.ncbi.nlm.nih.gov/25057632/
- Vaccine strain Polio circulation: https://www.npr.org/sections/goatsandsoda/2023/04/10/1168141163/the-dream-of-wiping-out-polio-might-need-a-rethink
- Lopalco, P L. “Wild and vaccine-derived poliovirus circulation, and implications for polio eradication.” Epidemiology and infection 145,3 (2017): 413-419. doi:10.1017/S0950268816002569 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9507676/
- Dhiman, Rachana et al. “Correlation between Non-Polio Acute Flaccid Paralysis Rates with Pulse Polio Frequency in India.” International journal of environmental research and public health 15,8 1755. 15 Aug. 2018, doi:10.3390/ijerph15081755 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6121585/
- Jaiswal, Nishant et al. “Equivalent schedules of intradermal fractional dose versus intramuscular full dose of inactivated polio vaccine for prevention of poliomyelitis.” The Cochrane database of systematic reviews 12,12 CD011780. 19 Dec. 2019, doi:10.1002/14651858.CD011780.pub2 https://pubmed.ncbi.nlm.nih.gov/31858595/
- Vilchez, Regis A, and Janet S Butel. “Emergent human pathogen simian virus 40 and its role in cancer.” Clinical microbiology reviews 17,3 (2004): 495-508, table of contents. doi:10.1128/CMR.17.3.495-508.2004 https://pubmed.ncbi.nlm.nih.gov/15258090/
- Petricciani, John et al. “Adventitious agents in viral vaccines: lessons learned from 4 case studies.” Biologicals : journal of the International Association of Biological Standardization 42,5 (2014): 223-36. doi:10.1016/j.biologicals.2014.07.003 https://pubmed.ncbi.nlm.nih.gov/25135887/
- Deischer TA, Doan NV, et al. Impact of environmental factors on the prevalence of autistic disorder after 1979. J Public Health Epidemiol 2014 Sep; 6(9): 271-86. https://academicjournals.org/article/article1411048618_Deisher%20et%20al.pdf
- Jarzyna, Peter et al. “Insertional mutagenesis and autoimmunity induced disease caused by human fetal and retroviral residual toxins in vaccines.” Issues in law & medicine 31,2 (2016): 221-234. https://pubmed.ncbi.nlm.nih.gov/29108182/
- Rose, Noel R. “Negative selection, epitope mimicry and autoimmunity.” Current opinion in immunology 49 (2017): 51-55. doi:10.1016/j.coi.2017.08.014 https://pubmed.ncbi.nlm.nih.gov/29102863/
- Fan, Hung, and Chassidy Johnson. “Insertional oncogenesis by non-acute retroviruses: implications for gene therapy.” Viruses 3,4 (2011): 398-422. doi:10.3390/v3040398 https://pubmed.ncbi.nlm.nih.gov/21994739/
- Sokol, Martin et al. “Novel principles of gamma-retroviral insertional transcription activation in murine leukemia virus-induced end-stage tumors.” Retrovirology 11 36. 19 May. 2014, doi:10.1186/1742-4690-11-36 https://pubmed.ncbi.nlm.nih.gov/24886479/
- O’Leary, Sean T et al. “The risk of immune thrombocytopenic purpura after vaccination in children and adolescents.” Pediatrics 129,2 (2012): 248-55. doi:10.1542/peds.2011-1111 https://pubmed.ncbi.nlm.nih.gov/22232308/
- Cecinati, Valerio et al. “Vaccine administration and the development of immune thrombocytopenic purpura in children.” Human vaccines & immunotherapeutics 9,5 (2013): 1158-62. doi:10.4161/hv.23601 https://pubmed.ncbi.nlm.nih.gov/23324619/
Hepatitis A
- Deischer TA, Doan NV, et al. Impact of environmental factors on the prevalence of autistic disorder after 1979. J Public Health Epidemiol 2014 Sep; 6(9): 271-86. https://academicjournals.org/article/article1411048618_Deisher%20et%20al.pdf
- Jarzyna, Peter et al. “Insertional mutagenesis and autoimmunity induced disease caused by human fetal and retroviral residual toxins in vaccines.” Issues in law & medicine 31,2 (2016): 221-234. https://pubmed.ncbi.nlm.nih.gov/29108182/
- Rose, Noel R. “Negative selection, epitope mimicry and autoimmunity.” Current opinion in immunology 49 (2017): 51-55. doi:10.1016/j.coi.2017.08.014 https://pubmed.ncbi.nlm.nih.gov/29102863/
- Fan, Hung, and Chassidy Johnson. “Insertional oncogenesis by non-acute retroviruses: implications for gene therapy.” Viruses 3,4 (2011): 398-422. doi:10.3390/v3040398 https://pubmed.ncbi.nlm.nih.gov/21994739/
- Sokol, Martin et al. “Novel principles of gamma-retroviral insertional transcription activation in murine leukemia virus-induced end-stage tumors.” Retrovirology 11 36. 19 May. 2014, doi:10.1186/1742-4690-11-36 https://pubmed.ncbi.nlm.nih.gov/24886479/
- O’Leary, Sean T et al. “The risk of immune thrombocytopenic purpura after vaccination in children and adolescents.” Pediatrics 129,2 (2012): 248-55. doi:10.1542/peds.2011-1111 https://pubmed.ncbi.nlm.nih.gov/22232308/
- Cecinati, Valerio et al. “Vaccine administration and the development of immune thrombocytopenic purpura in children.” Human vaccines & immunotherapeutics 9,5 (2013): 1158-62. doi:10.4161/hv.23601 https://pubmed.ncbi.nlm.nih.gov/23324619/
- “The Virus and the Vaccine” article by printed in The Atlantic
- “The Virus and the Vaccine” book by Debbie Bookchin & Jim Schumacher
- “Plague” book by Judy Mikovits
Influenza
- Rikin, Sharon et al. “Assessment of temporally-related acute respiratory illness following influenza vaccination.” Vaccine 36,15 (2018): 1958-1964. doi:10.1016/j.vaccine.2018.02.105 https://pubmed.ncbi.nlm.nih.gov/29525279/
- Cowling, Benjamin J et al. “Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine.” Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 54,12 (2012): 1778-83. doi:10.1093/cid/cis307 https://pubmed.ncbi.nlm.nih.gov/22423139/
- Joshi, Avni Y et al. “Effectiveness of trivalent inactivated influenza vaccine in influenza-related hospitalization in children: a case-control study.” Allergy and asthma proceedings 33,2 (2012): e23-7. https://pubmed.ncbi.nlm.nih.gov/22525386/
- Bodewes R, Kreijtz JH, et al. Vaccination against human influenza A/H3N2 virus prevents the induction of heterosubtypic immunity against lethal infection with Avian influenza A/H5N1 virus. PloS One 2009; 4(5): e5538 https://pubmed.ncbi.nlm.nih.gov/19440239/
- Skowronski DM, De Serres G, Crowcroft NS, Janjua NZ, Boulianne N, et al. (2010) Association between the 2008–09 Seasonal Influenza Vaccine and Pandemic H1N1 Illness during Spring–Summer 2009: Four Observational Studies from Canada. PLOS Medicine 7(4): e1000258. https://journals.plos.org/plosmedicine/article/citation?id=10.1371/journal.pmed.1000258
- Hayward, Andrew C et al. “Natural T Cell-mediated Protection against Seasonal and Pandemic Influenza. Results of the Flu Watch Cohort Study.” American journal of respiratory and critical care medicine 191,12 (2015): 1422-31. doi:10.1164/rccm.201411-1988OC https://pubmed.ncbi.nlm.nih.gov/25844934/
- Ohmit, Suzanne E et al. “Influenza vaccine effectiveness in the community and the household.” Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 56,10 (2013): 1363-9. doi:10.1093/cid/cit060 https://pubmed.ncbi.nlm.nih.gov/23413420/
- Wolff, Greg G. “Influenza vaccination and respiratory virus interference among Department of Defense personnel during the 2017-2018 influenza season.” Vaccine 38,2 (2020): 350-354. https://pubmed.ncbi.nlm.nih.gov/31607599/
- Bodewes, Rogier et al. “Annual vaccination against influenza virus hampers development of virus-specific CD8⁺ T cell immunity in children.” Journal of virology 85,22 (2011): 11995-2000. https://pubmed.ncbi.nlm.nih.gov/21880755/
- Dierig, Alexa et al. “Epidemiology of respiratory viral infections in children enrolled in a study of influenza vaccine effectiveness.” Influenza and other respiratory viruses 8,3 (2014): 293-301. https://pubmed.ncbi.nlm.nih.gov/24483149/
Hepatitis B
- Geier, David A, and Mark R Geier. “A case-control study of serious autoimmune adverse events following hepatitis B immunization.” Autoimmunity 38,4 (2005): 295-301. doi:10.1080/08916930500144484 https://pubmed.ncbi.nlm.nih.gov/16206512/
- Le Houézec, Dominique. “Evolution of multiple sclerosis in France since the beginning of hepatitis B vaccination.” Immunologic research 60,2-3 (2014): 219-25. doi:10.1007/s12026-014-8574-4 https://pubmed.ncbi.nlm.nih.gov/25395338/
- Hernán, Miguel A et al. “Recombinant hepatitis B vaccine and the risk of multiple sclerosis: a prospective study.” Neurology 63,5 (2004): 838-42. doi:10.1212/01.wnl.0000138433.61870.82 https://pubmed.ncbi.nlm.nih.gov/15365133/
- Mikaeloff, Yann et al. “Hepatitis B vaccine and the risk of CNS inflammatory demyelination in childhood.” Neurology 72,10 (2009): 873-80. doi:10.1212/01.wnl.0000335762.42177.07 https://pubmed.ncbi.nlm.nih.gov/18843097/
- Geier, D A, and M R Geier. “A one year followup of chronic arthritis following rubella and hepatitis B vaccination based upon analysis of the Vaccine Adverse Events Reporting System (VAERS) database.” Clinical and experimental rheumatology 20,6 (2002): 767-71. https://pubmed.ncbi.nlm.nih.gov/12508767/
- Crepeaux et al., 2015 Highly delayed systemic translocation of aluminum-based adjuvant in CD1 mice following intramuscular injections, Journal of Inorganic Biochemistry, 152:199-205. https://pubmed.ncbi.nlm.nih.gov/26384437/
- Crepeaux et al., 2017 Non-linear dose-response of aluminium hydroxide adjuvant particles: Selective low dose neurotoxicity, Toxicology, 375 (2017) 48–57 https://www.sciencedirect.com/science/article/abs/pii/S0300483X16303043
- Khan et al., 2013 Slow CCL2-dependent translocation of biopersistent particles from muscle to brain, BMC Medicine, 11:99 https://pubmed.ncbi.nlm.nih.gov/23557144/
- Guimarães, Luísa Eça et al. “Vaccines, adjuvants and autoimmunity.” Pharmacological research 100 (2015): 190-209. doi:10.1016/j.phrs.2015.08.003 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7129276/
- Tsumiyama, Ken et al. “Self-organized criticality theory of autoimmunity.” PloS one 4,12 e8382. 31 Dec. 2009, doi:10.1371/journal.pone.0008382 https://pubmed.ncbi.nlm.nih.gov/20046868/
Human Papilloma Virus
- National Cancer Institute: Cervical Cancer Stats
- CDC: Genital HPV Infection
- Komiyama M, Hasegawa K (2017) Comparison of Preventive Care for Cervical Cancer Between Japan And Western Countries: A Review. J Pharma Care Health Sys 4: 185. doi:10.4172/2376-0419.1000185
- CDC: Human Papillomavirus, Cervical Cancer Screening
- CDC: Cervical Cancer is Preventable
- CDC: Human Papillomavirus, Cervical Cancer Screening
- VAERS Data
- American College of Pediatricians – New Concerns about the Human Papillomavirus Vaccine
- Tomljenovic, Lucija, and Christopher A Shaw. “Human papillomavirus (HPV) vaccine policy and evidence-based medicine: are they at odds?.” Annals of medicine 45,2 (2013): 182-93. doi:10.3109/07853890.2011.645353 https://pubmed.ncbi.nlm.nih.gov/22188159/
- Tomljenovic, Lucija et al. “Human papillomavirus (HPV) vaccines as an option for preventing cervical malignancies: (how) effective and safe?.” Current pharmaceutical design 19,8 (2013): 1466-87. https://pubmed.ncbi.nlm.nih.gov/23016780/
- Tomljenovic, Lucija and C. A. Shaw. “Death after Quadrivalent Human Papillomavirus (HPV) Vaccination: Causal or Coincidental?” (2016).
- Martínez-Lavín, Manuel. “Hypothesis: Human papillomavirus vaccination syndrome–small fiber neuropathy and dysautonomia could be its underlying pathogenesis.” Clinical rheumatology 34,7 (2015): 1165-9. doi:10.1007/s10067-015-2969-z https://pubmed.ncbi.nlm.nih.gov/25990003/
- Gatto, Mariele et al. “Human papillomavirus vaccine and systemic lupus erythematosus.” Clinical rheumatology 32,9 (2013): 1301-7. doi:10.1007/s10067-013-2266-7 https://pubmed.ncbi.nlm.nih.gov/23624585/
- Brinth, Louise S et al. “Orthostatic intolerance and postural tachycardia syndrome as suspected adverse effects of vaccination against human papilloma virus.” Vaccine 33,22 (2015): 2602-5. doi:10.1016/j.vaccine.2015.03.098 https://pubmed.ncbi.nlm.nih.gov/25882168/
- Brinth, Louise et al. “Suspected side effects to the quadrivalent human papilloma vaccine.” Danish medical journal 62,4 (2015): A5064. https://pubmed.ncbi.nlm.nih.gov/25872549/
- Kinoshita, Tomomi et al. “Peripheral sympathetic nerve dysfunction in adolescent Japanese girls following immunization with the human papillomavirus vaccine.” Internal medicine (Tokyo, Japan) 53,19 (2014): 2185-200. doi:10.2169/internalmedicine.53.3133 https://pubmed.ncbi.nlm.nih.gov/25274229/
- Little, Deirdre Therese, and Harvey Rodrick Grenville Ward. “Adolescent Premature Ovarian Insufficiency Following Human Papillomavirus Vaccination: A Case Series Seen in General Practice.” Journal of investigative medicine high impact case reports 2,4 2324709614556129. 28 Oct. 2014, doi:10.1177/2324709614556129 https://pubmed.ncbi.nlm.nih.gov/26425627/
- Colafrancesco, Serena et al. “Human papilloma virus vaccine and primary ovarian failure: another facet of the autoimmune/inflammatory syndrome induced by adjuvants.” American journal of reproductive immunology (New York, N.Y. : 1989) 70,4 (2013): 309-16. doi:10.1111/aji.12151 https://pubmed.ncbi.nlm.nih.gov/23902317/
- Crepeaux et al., 2015 Highly delayed systemic translocation of aluminum-based adjuvant in CD1 mice following intramuscular injections, Journal of Inorganic Biochemistry, 152:199-205. https://pubmed.ncbi.nlm.nih.gov/26384437/
- Crepeaux et al., 2017 Non-linear dose-response of aluminium hydroxide adjuvant particles: Selective low dose neurotoxicity, Toxicology, 375 (2017) 48–57 https://www.sciencedirect.com/science/article/abs/pii/S0300483X16303043
- Khan et al., 2013 Slow CCL2-dependent translocation of biopersistent particles from muscle to brain, BMC Medicine, 11:99 https://pubmed.ncbi.nlm.nih.gov/23557144/
- Guimarães, Luísa Eça et al. “Vaccines, adjuvants and autoimmunity.” Pharmacological research 100 (2015): 190-209. doi:10.1016/j.phrs.2015.08.003 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7129276/
- Tsumiyama, Ken et al. “Self-organized criticality theory of autoimmunity.” PloS one 4,12 e8382. 31 Dec. 2009, doi:10.1371/journal.pone.0008382 https://pubmed.ncbi.nlm.nih.gov/20046868/
Live Viral Vaccines
Weakened strains of a virus are selected and grown on cells to produce live viral vaccines. Live viral vaccines produce active infections that are usually subclinical (asymptomatic), but can be spread to others. It is important that those who have just received a live viral vaccine avoid people whose immune systems are compromised as immunocompromised persons (such as those undergoing cancer treatment) may not be able to fight off even a weakened version of the virus.
How Are They Made?
Live viruses are grown on animal cells or cell lines derived from aborted human fetuses. The weakened versions of the virus are harvested from the cells to make the vaccine. These vaccines contain the weakened virus and some cell debris. The viruses are hard to purify because purification may inactivate them.
Pros
- Some confer long lasting immunity (~ 15 yrs).
- Induce a multi-faceted immune response.
Cons
- Recipients shed live virus that can infect others.1,2
- Presence of human fetal cell debris/DNA in MMR and chickenpox vaccines may result in autoimmunity.
- Vaccinated mothers do NOT pass sufficient antibodies to infants, leaving them vulnerable.
MMR (Measles, Mumps, Rubella)
The Illnesses:
- Measles: Measles is a mild childhood illness caused by an RNA virus that causes fever and characteristic rash that usually lasts about a week. Rarely, complications in susceptible individuals can lead to infection of the organs, and most concerningly, the brain. Infection results in lifelong immunity. Vitamin A plays a critical role in aiding the body to protect against this virus. In high-risk children with measles, studies have shown that bolus doses of Vitamin A can cut mortality in half.
- Mumps: Mumps is a mild childhood illness caused by an RNA virus that causes fever and characteristic rash that usually lasts about a week. The mumps virus preferentially affects the salivary glands, causing inflammation and swelling. This swelling of the cheeks is characteristic of those infected. Infection results in lifelong immunity. Rarely, complications in susceptible individuals can lead to infection of the organs. Infection in teenage boys can spread to the testis and compromise fertility.
- Rubella: Rubella is a mild childhood illness caused by an RNA virus that causes fever and rash that usually lasts about a week. Infection is generally so mild it goes unnoticed. However, if infection happens during the first trimester of pregnancy, it increases the risk of birth defects known as Congenital Rubella Syndrome (CRS). Even so, this syndrome is extremely rare.
Cost/Benefit: The rationale for instituting universal vaccination against measles and mumps was that it would reduce the number of sick days for children and prevent the severe complications that are a rare side of effect of teenagers contracting the illnesses. For Rubella, vaccination is seen as a way of ensuring pregnant mothers do not contract the illness, resulting in increased risk of birth defects. However, these few benefits come at a cost. Vaccine-generated immunity is NOT lifelong and begins to wear off after 15-20 years, whereas natural immunity is generally lifelong. Importantly, when children still contracted these viruses naturally, women were able to give “passive immunity” to their newborns which protected them from severe infection during their most vulnerable window (from birth to 2 years old). Vaccine-induced immunity is not strong enough to produce maternal immunity, leaving newborns susceptible to a potentially severe infection with one of these RNA viruses. Vaccination is usually not adequate to protect during this window because the infant immune system is NOT developmentally ready to generate a proper immune response to live viral vaccines, such as MMR, until children are well over 1 year old. Receipt of the vaccine that combines MMR and Varicella (MMRV-Proquad) is associated with a higher risk of seizures and hospitalization post-vaccination. Natural infection with RNA viruses such as measles has been reported to reduce the risk of several adult diseases, most notably, cancer.
Concerningly, these live 3 viral vaccines contain residual human cell debris and DNA/RNA from the cell lines they were grown in. The vaccines are grown in a cell line derived from an aborted male fetus (MCR5). This raises ethical concerns for many people. Medically, the presence of human cell debris can theoretically cause several problems. First, the cell debris and/or the genetic material may stimulate an aberrant immune response. Second, the residual DNA may be taken up by stem cells in the blood and insert into the host DNA, thereby causing insertional mutagenesis. The insertion of viral vaccine DNA into a person’s DNA increases the risk of cancers, specifically blood cancers such as leukemia and lymphoma. Third, residual human cell debris is often contaminated with human retroviruses, such as HERV-K, which are associated with chronic fatigue and cancer.
MMR Vaccine and Autism: In 1998, Dr. Andrew Wakefield published a study demonstrating a potential link between gastrointestinal problems and autism. The fact that he mentioned that many of the parents of his patients claimed that both the onset of the gastrointestinal problems and the regression into autism were co-incident with MMR vaccination eventually set off a firestorm of controversy. Following many anecdotal reports that their children regressed into autism following an MMR vaccine, the CDC commissioned a study to look at the relationship between the timing of the MMR vaccine and rates of autism. They published the results (DeStefano) in 2004 showing that boys vaccinated with the MMR vaccine prior to 36 months were 1.67 times and Black boys were 2.52 times more likely to be diagnosed with autism than those vaccinated after 36 months. However, later, the CDC Senior Scientist Dr. William Thompson came forward as a whistleblower, alleging that significant data were removed from the paper. Re-analysis of the allegedly removed data by Dr. Brian Hooker showed that Black boys were actually 3.86 times more likely to receive an autism diagnosis if they were vaccinated prior to 36 months. The are clear biological reasons that this difference might occur which are detailed in the “Key Susceptibilities” section of the VI-TA website.
The Vaccine: M-M-R®II by Merck is a live viral vaccine that is a combination of the measles, mumps and rubella viruses. A weakened version of the Ender’s attenuated Edmonton strain of the measles virus is grown on tissue culture derived from chick embryos. A weakened version of the Jeryl Lynn™ (B-level) strain of the mumps virus is grown on tissue culture derived from chick embryos. A weakened version of the rubella virus, known as Wistar RA 27/3 strain is grown on human cells derived from an aborted female fetus (WI-38 cells). Concerningly, in 2014, two Merck scientist whistleblowers came forward alleging that the mumps portion of the vaccine was ineffective and that they had been asked to falsify data and claim that it was still 95% effective when it was not effective.
As a result of being grown in cell culture, the final MMR vaccine product WILL contain residual chicken or human cell debris and DNA/RNA. It also contains gelatin and traces of the antibiotic neomycin, which can illicit allergic-type responses in some people. Contraindications include those with previous anaphylactic reactions to the vaccine and those who are immunocompromise. This is a live viral vaccine that sets up a subclinical infection in those who receive it. Some will manifest the clinical symptoms of rash. Thus, there is a risk of substantial infection in those who are immune-compromised and they should be avoided by those recently vaccinated with live viral vaccines, such as this one. Due to the length of time is has been available and the fact that it is a live viral vaccine that can cause an active infection, there are numerous contraindications to vaccination as well as reported cautions and adverse events listed in the vaccine insert that are detailed below.
Age Given: 1 year, and 5 years
Package Insert information can be found here. mmr_ii_pi (merck.com)
Vaccine Ingredients: M-M-R®II by Merck
- Weakened measles, mumps, rubella live viruses
- Sucrose and sorbitol (sugars)
- saline solution
- gelatin
- recombinant human albumin (blood protein)
- residual components of the human fetal lung tissue (WI-38)
- EDTA
- Neomycin (an antibiotic)
- trace quantities of cow fetus serum
As per the vaccine insert (M-M-R®II by Merck), vaccination is contraindicated under the following conditions:
4.1 Hypersensitivity Do not administer M-M-R II vaccine to individuals with a history of hypersensitivity to any component of the vaccine (including gelatin) {3} or who have experienced a hypersensitivity reaction following administration of a previous dose of M-M-R II vaccine or any other measles, mumps and rubella-containing vaccine. Do not administer M-M-R II vaccine to individuals with a history of anaphylaxis to neomycin [see Description (11)].
4.2 Immunosuppression Do not administer M-M-R II vaccine to individuals who are immunodeficient or immunosuppressed due to disease or medical therapy. Measles inclusion body encephalitis {4} (MIBE), pneumonitis {5} and death as a direct consequence of disseminated measles vaccine virus infection have been reported in immunocompromised individuals inadvertently vaccinated with measles-containing vaccine. In this population, disseminated mumps and rubella vaccine virus infection have also been reported.
4.3 Moderate or Severe Febrile Illness Do not administer M-M-R II vaccine to individuals with an active febrile illness with fever >101.3°F (>38.5°C).
4.4 Active Untreated Tuberculosis Do not administer M-M-R II vaccine to individuals with active untreated tuberculosis (TB).
4.5 Pregnancy Do not administer M-M-R II to individuals who are pregnant or who are planning on becoming pregnant within the next month [see Use in Specific Populations (8.1) and Patient Counseling Information (17)].
The vaccine insert (M-M-R®II by Merck), gives the following warning and precautions:
5.1 Febrile Seizure There is a risk of fever and associated febrile seizure in the first 2 weeks following immunization with M-M-R II vaccine. For children who have experienced a previous febrile seizure (from any cause) and those with a family history of febrile seizures there is a small increase in risk of febrile seizure following receipt of M-M-R II vaccine [see Adverse Reactions (6)].
5.2 Hypersensitivity to Eggs Individuals with a history of anaphylactic, anaphylactoid, or other immediate reactions (e.g., hives, swelling of the mouth and throat, difficulty breathing, hypotension, or shock) subsequent to egg ingestion may be at an enhanced risk of immediate-type hypersensitivity reactions after receiving M-M-R II vaccine. The potential risks and known benefits should be evaluated before considering vaccination in these individuals.
5.3 Thrombocytopenia Transient thrombocytopenia has been reported within 4-6 weeks following vaccination with measles, mumps and rubella vaccine. Carefully evaluate the potential risk and benefit of vaccination in children with thrombocytopenia or in those who experienced thrombocytopenia after vaccination with a previous dose of measles, mumps, and rubella vaccine {6-8} [see Adverse Reactions (6)].
5.4 Family History of Immunodeficiency Vaccination should be deferred in individuals with a family history of congenital or hereditary immunodeficiency until the individual’s immune status has been evaluated and the individual has been found to be immunocompetent. 4
5.5 Immune Globulins and Transfusions Immune Globulins (IG) and other blood products should not be given concurrently with M-M-R II [see Drug Interactions (7.2)]. These products may contain antibodies that interfere with vaccine virus replication and decrease the expected immune response. The Advisory Committee on Immunization Practices (ACIP) has specific recommendations for intervals between administration of antibody containing products and live virus vaccines.
As per the vaccine insert (M-M-R®II by Merck), vaccination is an association with vaccination and the following conditions:
6 ADVERSE REACTIONS The following adverse reactions include those identified during clinical trials or reported during post approval use of M-M-R II vaccine or its individual components.
Body as a Whole Panniculitis; atypical measles; fever; headache; dizziness; malaise; irritability. Cardiovascular System Vasculitis. Digestive System Pancreatitis; diarrhea; vomiting; parotitis; nausea.
Hematologic and Lymphatic Systems Thrombocytopenia; purpura; regional lymphadenopathy; leukocytosis.
Immune System Anaphylaxis, anaphylactoid reactions, angioedema (including peripheral or facial edema) and bronchial spasm.
Musculoskeletal System Arthritis; arthralgia; myalgia. Nervous System Encephalitis; encephalopathy; measles inclusion body encephalitis (MIBE) subacute sclerosing panencephalitis (SSPE);
Guillain-Barré Syndrome (GBS); acute disseminated encephalomyelitis (ADEM); transverse myelitis; febrile convulsions; afebrile convulsions or seizures; ataxia; polyneuritis; polyneuropathy; ocular palsies; paresthesia; syncope.
Respiratory System Pneumonia; pneumonitis; sore throat; cough; rhinitis.
Skin Stevens-Johnson syndrome; acute hemorrhagic edema of infancy;
Henoch-Schönlein purpura; erythema multiforme; urticaria; rash; measles-like rash; pruritus; injection site reactions (pain, erythema, swelling and vesiculation).
Special Senses — Ear Nerve deafness; otitis media. Special Senses — Eye Retinitis; optic neuritis; papillitis; conjunctivitis. Urogenital System Epididymitis; orchitis
The Illness: Chickenpox is caused by the Varicella virus. Infection causes fever and spots.
It can be diagnosed by the characteristic spots or a blood test and is transmitted like the common cold. Prior to the mid 1990s there were about 3.5 million cases each year in the US. Now there are about 50,000 reported cases each year. Chickenpox is NOT generally serious. However, it is more uncomfortable for adults than children. It is more serious for people with compromised immune systems and it can cause birth defects if contracted during pregnancy. There is an antiviral medication available, but for most cases, the infection is allowed to run it’s course.
Cost/Benefit: The rationale for instituting universal vaccination against chickenpox was that it would reduce the number of sick days for children and prevent the severe complications that are a rare side of effect of teenagers contracting the illness. However, these few benefits come at a cost. Vaccine-generated immunity is NOT lifelong and begins to wear off after 15-20 years, whereas natural immunity is generally lifelong. Moreover, when children still contracted natural chickenpox and exposed their parents and grandparents to the virus (again), it had the effect of “boosting” the immunity of the elders, thus preventing the varicella virus from re-emerging in their bodies as Shingles. Without children with chickenpox to boost the immunity of the elderly, we are seeing an epidemic of Shingles in adults. Due to the fact that the vaccine doesn’t provide as long-lasting immunity as natural immunity, children vaccinated against chickenpox appear to be more susceptible to Shingles infection as well. Thus, we are seeing an increase in young adults and even children with Shingles. Shingles is a more severe illness, manifesting as painful clusters of blisters and postherpetic neuralgia—sever and debilitating pain that can last for weeks, months or years. Thus, we are substituting a painful, debilitating illness for a mild illness that produces robust natural immunity, a dubious tradeoff. Vaccination against chickenpox is associated with higher risks of allergies and a rare blood disorder, called thrombocytopenia. Receipt of the vaccine that combines MMR and Varicella (MMRV-Proquad) is associated with a higher risk of seizures and hospitalization postvaccination. Natural infection with viruses such as chickenpox also reduces the risk of several adult diseases, most notably, cancer.
Concerningly, Chickenpox vaccines contain residual human cell debris and DNA/RNA from the cell line they were grown in. The vaccine is grown in a cell line derived from an aborted male fetus (MCR5). This raises ethical concerns for many people. Medically, the presence of human cell debris can theoretically cause several problems. First, the cell debris and/or the genetic material may bind to Toll-like receptors and activate an aberrant immune response. Second, the residual DNA may be taken up by stem cells in the blood and insert into the host DNA, thereby causing insertional mutagenesis. Insertional mutagenesis will increase the risk of cancers, specifically blood cancers such as leukemia and lymphoma. Third, residual human cell debris is often contaminated with human retroviruses, such as HERV-K, which are associated with chronic fatigue and cancer.
The Vaccine: VARIVAX by Merck) is a live viral vaccine. A weakened version of the chickenpox (varicella) virus is grown on human cells derived for an aborted male fetus (MCR5 cells) to produce the virus. As a result of being grown in human cells, the final vaccine product WILL contain residual human cell debris and DNA/RNA. It also contains monosodium glutamate, gelatin and traces of the antibiotic neomycin, which can both illicit allergic-type responses in some people. Contraindications include those with previous anaphylactic reactions to the vaccine and those who are immunocompromise. This is a live viral vaccine and sets up a subclinical infection in those who receive it. Some will manifest the clinical symptoms of rash. Thus, there is a risk of substantial infection in those who are immunocompromised and they should be avoided by those recently vaccinate with live viral vaccines, such as this one.
Age Given: 1 year, and 5 years
Package Insert information can be found here. https://www.immunize.org/fda/#var
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
Vaccine Ingredients: Varivax
- live virus
- sucrose
- saline solution
- gelatin
- monosodium glutamate
- potassium
- residual components of the Merck human diploid cells (MRC-5)
- EDTA
- neomycin
- trace quantities of cow fetus serum
The Illness: Rotavirus is the most common cause of severe diarrhea in kids under two years old. Infection with Rotavirus can result in dehydration as the diarrhea can last more than a few days. IV rehydration is used to treat the dehydration. Rotavirus is transmitted by contact with the stools or saliva of an infected person. Rotavirus is resistant to common disinfectants and antibacterial hand soaps. A strong antiseptic or alcohol is needed to destroy the virus.
Cost/Benefit Analysis: Rotavirus is most severe in the first year of life, but less problematic after that. Children who are breastfed and not in daycare are at low risk during their first year of life, when risk of severe infection and hospitalization is highest. In the U.S, about 50,000 children per year are hospitalized for rotavirus in order to receive proper hydration. Both vaccines currently on the market are live viral vaccines. Live viral vaccines generally give robust long-term immunity. However, the live Rotavirus vaccine creates an active infection in the gut in order to establish immunity. This could be problematic for those with weakened immune systems. The vaccine strain virus is also shed in the stool and can be passed to others in the household or environment. Both currently available versions of the vaccine are associated with a low risk of intussusception (part of the intestine folding into itself creating a blockage), Kawasaki Disease, Hematochezia (gastrointestinal bleed) and seizure. Current Rotavirus vaccines are live viral vaccines that are grown in VERO (monkey kidney cells that lack anti-viral defenses). VERO cells are known to potentially contain a variety of monkey viruses, retroviruses and possibly other viruses that the cell line may have been used to propagate. Thus, there is a substantial concern that the vaccine may be contaminated with adventitious (unintentional) viruses that could infect the child.
As per the vaccine insert (Rotarix), vaccination is contraindicated under the following conditions:
4.1 Hypersensitivity A demonstrated history of hypersensitivity to any component of the vaccine. Infants who develop symptoms suggestive of hypersensitivity after receiving a dose of ROTARIX should not receive further doses of ROTARIX.
4.2 Gastrointestinal Tract Congenital Malformation Infants with a history of uncorrected congenital malformation of the gastrointestinal tract (such as Meckel’s diverticulum) that would predispose the infant for intussusception should not receive ROTARIX.
4.3 History of Intussusception Infants with a history of intussusception should not receive ROTARIX [see Warnings and Precautions (5.5)]. In postmarketing experience, intussusception resulting in death following a second dose has been reported following a history of intussusception after the first dose [see Adverse Reactions (6.2)].
4.4 Severe Combined Immunodeficiency Disease Infants with Severe Combined Immunodeficiency Disease (SCID) should not receive ROTARIX. Postmarketing reports of gastroenteritis, including severe diarrhea and prolonged shedding of vaccine virus, have been reported in infants who were administered live, oral rotavirus vaccines and later identified as having SCID
Insert Data on risk of Intussusception continues:
5.5 Intussusception Following administration of a previously licensed oral live rhesus rotavirus-based vaccine, an increased risk of intussusception was observed.1
The risk of intussusception with ROTARIX was evaluated in a pre-licensure randomized, placebo-controlled safety study (including 63,225 infants) conducted in Latin America and Finland. No increased risk of intussusception was observed in this clinical trial following administration of ROTARIX when compared with placebo. [See Adverse Reactions (6.1).]
In a postmarketing, observational study conducted in Mexico, cases of intussusception were observed in temporal association within 31 days following the first dose of ROTARIX, with a clustering of cases in the first 7 days. [See Adverse Reactions (6.2).]
Other postmarketing observational studies conducted in Brazil and Australia also suggest an increased risk of intussusception within the first 7 days following the second dose of ROTARIX.2,3 [See Adverse Reactions (6.2).]
In worldwide passive postmarketing surveillance, cases of intussusception have been reported in temporal association with ROTARIX [see Adverse Reactions (6.2)].
The Vaccines: Rotavirus vaccines are made by growing rotaviruses on VERO (monkey kidney) cells that lack anti-viral defenses. In the past, VERO cells have been shown to be contaminated with many monkey viruses as well as retroviruses.
Age Given: 2 months, 4 months, and 6 months
Package Insert information can be found here. https://www.immunize.org/fda/#rota
Information concerning adverse events can be found in section 6, which contains information on the clinical trials as well as the post-marketing data.
Vaccine Ingredients: RotaTeq (Merck)
- five virus strains, live and whole
- sucrose (a sugar)
- sodium citrate, phosphate, and hydroxide
- ploysorbate 80
- cell culture media
- traces of fetal cow blood
Vaccine Ingredients: Rotarix (GlaxoSmithKline)
- Single strain virus, live and whole
- sucrose (a sugar), dextran (a sugar), sorbitol (a sugar)
- Amino acids
- sodium citrate, phosphate, and hydroxide
- calcium carbonate
- xanthum gum
- cell culture media (DMEM)
- sterile water
References
General to Live Viral Vaccines
1. Deischer TA, Doan NV, et al. Impact of environmental factors on the prevalence of autistic disorder after 1979. J Public Health Epidemiol 2014 Sep; 6(9): 271-86. https://academicjournals.org/article/article1411048618_Deisher%20et%20al.pdf
2. Jarzyna, Peter et al. “Insertional mutagenesis and autoimmunity induced disease caused by human fetal and retroviral residual toxins in vaccines.” Issues in law & medicine vol. 31,2 (2016): 221-234. https://pubmed.ncbi.nlm.nih.gov/29108182/
3. Rose, Noel R. “Negative selection, epitope mimicry and autoimmunity.” Current opinion in immunology vol. 49 (2017): 51-55. doi:10.1016/j.coi.2017.08.014 https://pubmed.ncbi.nlm.nih.gov/29102863/
4. Fan, Hung, and Chassidy Johnson. “Insertional oncogenesis by non-acute retroviruses: implications for gene therapy.” Viruses vol. 3,4 (2011): 398-422. doi:10.3390/v3040398 https://pubmed.ncbi.nlm.nih.gov/21994739/
5. Sokol, Martin et al. “Novel principles of gamma-retroviral insertional transcription activation in murine leukemia virus-induced end-stage tumors.” Retrovirology vol. 11 36. 19 May. 2014, doi:10.1186/1742-4690-11-36 https://pubmed.ncbi.nlm.nih.gov/24886479/
6. O’Leary, Sean T et al. “The risk of immune thrombocytopenic purpura after vaccination in children and adolescents.” Pediatrics vol. 129,2 (2012): 248-55. doi:10.1542/peds.2011-1111 https://pubmed.ncbi.nlm.nih.gov/22232308/
7. Cecinati, Valerio et al. “Vaccine administration and the development of immune thrombocytopenic purpura in children.” Human vaccines & immunotherapeutics vol. 9,5 (2013): 1158-62. doi:10.4161/hv.23601 https://pubmed.ncbi.nlm.nih.gov/23324619/
8. Albonico, H U et al. “Febrile infectious childhood diseases in the history of cancer patients and matched controls.” Medical hypotheses vol. 51,4 (1998): 315-20. doi:10.1016/s0306-9877(98)90055-x https://pubmed.ncbi.nlm.nih.gov/9824838/
ChickenPox
9. Wrensch, Margaret et al. “History of chickenpox and shingles and prevalence of antibodies to varicella-zoster virus and three other herpesviruses among adults with glioma and controls.” American journal of epidemiology vol. 161,10 (2005): 929-38. doi:10.1093/aje/kwi119 https://pubmed.ncbi.nlm.nih.gov/15870157/
10. Leske, Henning et al. “Varicella zoster virus infection of malignant glioma cell cultures: a new candidate for oncolytic virotherapy?.” Anticancer research vol. 32,4 (2012): 1137-44. https://pubmed.ncbi.nlm.nih.gov/22493342/
Rotavirus
11. Carlin, John B et al. “Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program.” Clinical infectious diseases : an official publication of the Infectious Diseases Society of America vol. 57,10 (2013): 1427-34. doi:10.1093/cid/cit520 https://pubmed.ncbi.nlm.nih.gov/23964090/
12. Patel, Manish M et al. “Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil.” The New England journal of medicine vol. 364,24 (2011): 2283-92. doi:10.1056/NEJMoa1012952 https://pubmed.ncbi.nlm.nih.gov/21675888/
MMR
13. Klein, Nicola P et al. “Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures.” Pediatrics vol. 126,1 (2010): e1-8. doi:10.1542/peds.2010-0665 https://pubmed.ncbi.nlm.nih.gov/20587679/
14. MacDonald, Shannon E et al. “Risk of febrile seizures after first dose of measles-mumps-rubella-varicella vaccine: a population-based cohort study.” CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne vol. 186,11 (2014): 824-9. doi:10.1503/cmaj.140078 https://pubmed.ncbi.nlm.nih.gov/24914115/
15. Schink, Tania et al. “Risk of febrile convulsions after MMRV vaccination in comparison to MMR or MMR+V vaccination.” Vaccine vol. 32,6 (2014): 645-50. doi:10.1016/j.vaccine.2013.12.011 https://pubmed.ncbi.nlm.nih.gov/24374498/
16. Jacobsen, Steven J et al. “Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting.” Vaccine vol. 27,34 (2009): 4656-61. doi:10.1016/j.vaccine.2009.05.056 https://pubmed.ncbi.nlm.nih.gov/19520201/
17. Schultz, Stephen T et al. “Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic disorder: the results of a parent survey.” Autism : the international journal of research and practice vol. 12,3 (2008): 293-307. doi:10.1177/1362361307089518 18. https://pubmed.ncbi.nlm.nih.gov/18445737/
19. Parker, William et al. “The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism.” The Journal of international medical research vol. 45,2 (2017): 407-438. doi:10.1177/0300060517693423 https://pubmed.ncbi.nlm.nih.gov/28415925/
20. Singh, V.K., Lin, S.X., Newell, E. et al. Abnormal measles-mumps-rubella antibodies and CNS autoimmunity in children with autism. J Biomed Sci 9, 359–364 (2002). https://link.springer.com/article/10.1007/BF02256592
21. Singh, Vijendra K, and Ryan L Jensen. “Elevated levels of measles antibodies in children with autism.” Pediatric neurology vol. 28,4 (2003): 292-4. doi:10.1016/s0887-8994(02)00627-6 https://pubmed.ncbi.nlm.nih.gov/12849883/
22. Wakefield, A J et al. “Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children.” Lancet (London, England) vol. 351,9103 (1998): 637-41. doi:10.1016/s0140-6736(97)11096-0 retracted https://pubmed.ncbi.nlm.nih.gov/9500320/
23. Wakefield, Andrew Callous Disregard: Autism and Vaccines—The Truth Behind a Tragedy (New York: Skyhorse Publishing, 2017), ISBN: 9781510729667
24. DeStefano, Frank et al. “Age at first measles-mumps-rubella vaccination in children with autism and school-matched control subjects: a population-based study in metropolitan Atlanta.” Pediatrics vol. 113,2 (2004): 259-66. doi:10.1542/peds.113.2.259 https://pubmed.ncbi.nlm.nih.gov/14754936/
25. Thompson, N P et al. “Is measles vaccination a risk factor for inflammatory bowel disease?.” Lancet (London, England) vol. 345,8957 (1995): 1071-4. doi:10.1016/s0140-6736(95)90816-1 https://pubmed.ncbi.nlm.nih.gov/7715338/
26. Lavy, A et al. “Measles is more prevalent in Crohn’s disease patients. A multicentre Israeli study.” Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver vol. 33,6 (2001): 472-6. doi:10.1016/s1590-8658(01)80024-4 https://pubmed.ncbi.nlm.nih.gov/11572573/
How Do I Make A Change?
Who is really healthier, the vaccinated or the unvaccinated? What does the science REALLY show? Find out more in the new book from Dr. Brian Hooker “Vax-Unvax: Let The Science Speak.”
JOIN VI-TA
Learn more about how you can support the health and development of your growing child as well as address common childhood illnesses
(for less than the price of 2 cups of coffee a month)
Aren’t Vaccines Required For School?
Vaccine Exemptions
All states allow vaccine exemptions for school attendance. There are three types of exemptions that can be obtained:
- Medical
- Religious
- Philosophical
The pictorial shows which exemptions were available in each state as of the year 2019. Since that time, Mississippi has re-established a religious exemption. To obtain a waiver/exemption for school, it is usually necessary to visit your local health department and request one. A medical exemption requires a doctor’s statement and can be difficult to obtain in many states. You can learn more about exemptions at this website.
Many states have organizations that promote awareness of vaccine issues and their websites contain information on obtaining exemptions that is specific to that state. Several are listed below.
Alabama
Alaska
Arizona
Arkansas
California
California Coalition for Vaccine Choice
Delaware
Hawaii
Idaho
Indiana
Kansas
Louisiana
Massachusetts
Montana
Montanans for Medical Freedom Coalition
Nebraska
Nevada
New Hampshire
New Mexico
North Carolina
Citizens for Healthcare Freedom
North Dakota
Oregon
South Carolina
South Dakota
Tennessee
Utah
Vermont
Virginia
West Virginia
Wisconsin
Wyoming
Wyoming
Want to learn more about how you can recover your health?
Become a VI-TA member
(It's about the same as the price of 1 cup of Starbucks coffee!)
Medical Disclaimer: The information at vi-ta.org is presented for informational purposes only. Nothing in this website constitutes medical advice and is not intended to diagnose or prescribe for any medical or psychological condition, nor to prevent, treat, mitigate or cure such conditions. Parents, caregivers, consumers, patients, individuals and health practitioners must use their own judgment concerning specific treatment options.
vi-ta.org authors, editors, principals, partners and affiliates disclaim any liability or responsibility to any person or organization for any loss, damage, expense, fine, injury, or penalty that may arise or result from the use of any information, recommendations, opinions and/or errors on this website or in our articles, emails or links. Any use of, or reliance on, information reflected on this website, emails, articles or references is solely the responsibility of the viewer.
vi-ta.org encourages you to make your own health care decisions based on your judgment and research in partnership with a qualified healthcare professional. These statements have not been evaluated by the Food and Drug Administration. The information on this website is not intended to diagnose, treat, cure or prevent any disease.
Inflammation
- One of the main ways that vaccines cause injury is by stimulating unchecked inflammation. Vaccines are designed to cause an inflammatory immune response. People can be susceptible to run-away inflammation due to any, or a combination of factors, including:
- Genetic or epigenetic differences,
- Low vitamin D activity
- Low anti-oxidant (glutathione/vitamin C) status
- Toxin overload
- Compromised ability to detox
Calming Inflammation
Approaches to calming inflammation include:
- Reduce sugar and simple carbohydrates
- Increase anti-oxidants in diet or through supplementation
- Ensure adequate Vitamin D activity through:
- Cool, morning sun exposure
- Intake sufficient Vitamin D, A, K and magnesium in diet/supplementation
- Increase microbiome diversity
- Probiotics/Prebiotics
- Fermented foods
- Heal leaky gut
- GAPS diet
- Bone broth and Collagen
Key Inflammation Studies
- Pourcyrous, M et al. “Interleukin-6, C-reactive protein, and abnormal cardiorespiratory responses to immunization in premature infants.” Pediatrics vol. 101,3 (1998): E3. doi:10.1542/peds.101.3.e3 https://pubmed.ncbi.nlm.nih.gov/9481022/
- DeMeo, Stephen D et al. “Adverse Events After Routine Immunization of Extremely Low-Birth-Weight Infants.” JAMA pediatrics vol. 169,8 (2015): 740-5. doi:10.1001/jamapediatrics.2015.0418 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4523398/
- Pourcyrous, Massroor et al. “Primary immunization of premature infants with gestational age <35 weeks: cardiorespiratory complications and C-reactive protein responses associated with administration of single and multiple separate vaccines simultaneously.” The Journal of pediatrics vol. 151,2 (2007): 167-72. doi:10.1016/j.jpeds.2007.02.059 https://pubmed.ncbi.nlm.nih.gov/17643770/
- Poggi, Chiara, and Carlo Dani. “Sepsis and Oxidative Stress in the Newborn: From Pathogenesis to Novel Therapeutic Targets.” Oxidative medicine and cellular longevity vol. 2018 9390140. 2 Aug. 2018, doi:10.1155/2018/9390140 https://pubmed.ncbi.nlm.nih.gov/30174784/
- Naik, Usha S et al. “A study of nuclear transcription factor-kappa B in childhood autism.” PloS one vol. 6,5 e19488. 9 May. 2011, doi:10.1371/journal.pone.0019488 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3090385/
- Young, Adam M H et al. “Aberrant NF-kappaB expression in autism spectrum condition: a mechanism for neuroinflammation.” Frontiers in psychiatry vol. 2 27. 13 May. 2011, doi:10.3389/fpsyt.2011.00027 https://pubmed.ncbi.nlm.nih.gov/21629840/
Excessive Prenatal/Infant Immune Activation
- A significant and growing body of data indicate that the prenatal and infant brain are uniquely susceptible to injury from aberrant or excessive immune activation either in the pregnant mother or in the infant. This is likely because during this period of time the fetal/infant brain is undergoing synaptogenesis (formation of synapse connections between neurons). This type of early immune activation has been established to be causal in the development of autism and other neurodevelopmental disorders.
- Factors that produce this type of immune activation are under investigation and include severe infection, and injection of ‘poly-IC’ or LPS (which mimic bacterial infection). These factors induce the expression of inflammatory cytokines, and the greater the duration and intensity of cytokine expression, the higher the risk of autism. Chronic upregulation of inflammatory cytokines is also associated with a greater risk of autism. However, there is no evidence of normal cytokine activation, resulting from normal illness (in mother or child), increasing autism risk.
Avoiding Excessive Prenatal/Infant Immune Activation
- Very rarely, a mother will come down with a severe infection that can cause this type of immune activation. However, the most likely cause of excessive prenatal immune activation is the mother getting vaccinated while pregnant.
- Carefully spacing and putting off all unnecessary vaccines until the child is older than 2-3 yrs will significantly reduce the amount of immune activation that might have a deleterious impact on the development of the child’s nervous system.
It’s critical and an infant’s system remain ANTI-INFLAMMATORY during brain development
Key Studies on Prenatal/Infant Immune Activation
- Meyer et al., 2006 The Time of Prenatal Immune Challenge Determines the Specificity of Inflammation-Mediated Brain and Behavioral Pathology, The Journal of Neuroscience, 26(18):4752– 4762.
- Meyer et al., 2007 The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse?, Neuroscientist, Jun;13(3):241-56.
- Meyer et al., 2009 In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders, Neuroscience and Biobehavioral Reviews, 33 (2009) 1061–1079.
- Meyer 2014, Prenatal Poly(I:C) Exposure and Other Developmental Immune Activation Models in Rodent Systems, Biological Psychiatry, 75:307-315
- Estes and McAllister, 2016 Maternal immune activation: implications for neuropsychiatric disorders, Science, 353 (6301) 772-777.
- Careaga et al 2017 Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates, Biological Psychiatry, March 1, 2017; 81:391–401
- Atladottir et al., Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders, Journal of Autism and Developmental Disorders, 2010 Dec;40(12):1423-1430
- Jones et al., 2016 Autism with Intellectual Disability is Associated with Increased Levels of Maternal Cytokines and Chemokines During Gestation, Molecular Psychiatry, 22(2):273-279.
- Zerbo, O., Qian, Y., Yoshida, C., Fireman, B. H., Klein, N. P., Croen, L. A. (2017). Association between influenza infection and vaccination during pregnancy
Aluminum
- Aluminum is a known neurotoxin
- Recent scientific studies are showing that the aluminum in vaccines is more problematic than was previously thought. Taken together, studies from experts all over the world create a model in which aluminum adjuvant-bound antigen is ingested by white blood cells (macrophages) and carried to remote areas of the body including lymph nodes, spleen, liver and the brain, where it causes chronic inflammation.
- Aluminum strongly induces the expression of the inflammatory cytokine interleukin-6 (IL-6) in the brain, suggesting this may be an important mechanism by which it causes neurologic damage. Lastly, in 2018, Dr. Chris Exley found that brains of autistic people have some of the highest levels of aluminum that he has ever seen in human brains.
Dealing with Aluminum Toxicity
- Dr. Chris Exley has done experiments demonstrating that drinking mineral water high in silica (from volcanic groundwater) can be used to safely chelated out aluminum. Brands available in the U.S. include Volvic and Fiji. You cannot put powdered silica into water to “make” your own “silica water.”
- Calming Inflammation can be Helpful:
- Reduce sugar and simple carbohydrates
- Increase anti-oxidants in diet or through supplementation
- Ensure adequate Vitamin D activity through:
- Cool, morning sun exposure
- Intake sufficient Vitamin D, A, K and magnesium in diet/supplementation
Infants vaccinated according to the CDC schedule spend 70% of their first 7 months in a state of aluminum toxicity (black), while infants vaccinated according to the “Vaccine Friendly Plan” only spent 5% of that time in aluminum toxicity (green). (4)
Key Studies on Aluminum
- Petrik, M. S., Wong, M. C., Tabata, R. C., Garry, R. F., & Shaw, C. A. (2007). Aluminum adjuvant linked to Gulf War illness induces motor neuron death in mice. Neuromolecular Medicine, 9(1), 83–100. https://doi.org/10.1385/NMM:9:1:83
- Shaw, Christopher A, and Michael S Petrik. “Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration.” Journal of inorganic biochemistry vol. 103,11 (2009): 1555-62. doi:10.1016/j.jinorgbio.2009.05.019 https://pubmed.ncbi.nlm.nih.gov/19740540/
- Shaw, C A et al. “Administration of aluminium to neonatal mice in vaccine-relevant amounts is associated with adverse long term neurological outcomes.” Journal of inorganic biochemistry vol. 128 (2013): 237-44. doi:10.1016/j.jinorgbio.2013.07.022 https://pubmed.ncbi.nlm.nih.gov/23932735/
- McFarland, G., La Joie, E., Thomas, P, Lyons-Weiler (2019). Acute exposure and chronic retention of aluminum in three vaccine schedules and effects of genetic and environmental variation. Journal of Trace Elements in Medicine and Biology, March 2020. https://www.sciencedirect.com/science/article/pii/S0946672X19305784
- Thomas, P., & Margulis, J. (2016). The vaccine-friendly plan: Dr. Paul’s safe and effective approach to immunity and health — from pregnancy through your child’s teen years. New York, NY: Ballantine Books
- Bilkei-Gorzó, A. “Neurotoxic effect of enteral aluminium.” Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association vol. 31,5 (1993): 357-61. doi:10.1016/0278-6915(93)90191-z https://pubmed.ncbi.nlm.nih.gov/8505021/
- Miller, N. Z. (2016b). Aluminum in childhood vaccines is unsafe. Journal of American Physicians and Surgeons, 21(4), 109–117. Retrieved from http://jpands.org/vol21no4/miller.pd
- Crepeaux et al., 2015 Highly delayed systemic translocation of aluminum-based adjuvant in CD1 mice following intramuscular injections, Journal of Inorganic Biochemistry, 152:199-205. https://pubmed.ncbi.nlm.nih.gov/26384437/
- Crepeaux et al., 2017 Non-linear dose-response of aluminium hydroxide adjuvant particles: Selective low dose neurotoxicity, Toxicology, 375 (2017) 48–57 https://www.sciencedirect.com/science/article/abs/pii/S0300483X16303043
- Shaw, C. A., Seneff, S., Kette, S. D., Tomljenovic, L., Oller, J. W., & Davidson, R. M. (2014). Aluminum-induced entropy in biological systems: implications for neurological disease. Journal of Toxicology, 2014. https://doi.org/10.1155/2014/491316 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4202242/
- Khan et al., 2013 Slow CCL2-dependent translocation of biopersistent particles from muscle to brain, BMC Medicine, 11:99 https://pubmed.ncbi.nlm.nih.gov/23557144/
- Mold, M., Umar, D., King, A., & Exley, C. (2017). Aluminium in brain tissue in autism. Journal of Trace Elements in Medicine and Biology. https://doi.org/10.1016/j.jtemb.2017.11.01 https://pubmed.ncbi.nlm.nih.gov/29413113/
Autoimmunity
- Vaccines have the ability to stimulate the body to attack its own tissues by two general mechanisms.
- Vaccines can cause chronic activation of the immune system, leading to dysregulated antibody
production, which allows the production and persistence of antibodies made to “self,” as well as
T-cells which attack the bodies tissues. - Live viral vaccines contain human cell debris that the body is stimulated to make antibodies
against. These antibodies then go on to attack the body’s own tissues. - Genetic variations that predispose to autoimmunity include specific variants of MCH class I and II.
Dealing with Autoimmunity
- Autoimmunity can be one of the most difficult manifestations of vaccine injury to deal with because your immune system is producing antibodies and/or immune cells that are attacking your own tissues. Therapeutic approaches include:
- IVIG- this is an expensive therapy that is hard to get insurance to cover in which the blood of thousands of individuals is concentrated and put into your body through an IV. The product is able to bind and neutralize autoantibodies and has non-specific anti-inflammatory and immune modulating effects.
- Healing Leaky Gut- When a person has “leaky gut,” molecules are leaking out of the guy into the bloodstream, resulting in chronic immune activation every time they eat. Chronic immune activation can lead to or exacerbate T-cell dysregulation that leads to autoimmune responses. Thus, healing the gut can reduce the chronic immune activation and allow re-modulation of immune response.
- Shutting down viral re-activation and other infections-Chronic infections of any kind result in chronic immune activation. If infections can be identified and effectively dealt with, autoimmune responses may fade away.
- Reduce Inflammation-because inflammation exacerbates chronic immune activation, reducing inflammation is key in any treatment of autoimmunity.
Key Studies on Autoimmunity
- Jarzyna, Peter et al. “Insertional mutagenesis and autoimmunity induced disease caused by human fetal and retroviral residual toxins in vaccines.” Issues in law & medicine vol. 31,2 (2016): 221-234. https://pubmed.ncbi.nlm.nih.gov/29108182/
- Rose, Noel R. “Negative selection, epitope mimicry and autoimmunity.” Current opinion in immunology vol. 49 (2017): 51-55. doi:10.1016/j.coi.2017.08.014 https://pubmed.ncbi.nlm.nih.gov/29102863/
- Guimarães, Luísa Eça et al. “Vaccines, adjuvants and autoimmunity.” Pharmacological research vol. 100 (2015): 190-209. doi:10.1016/j.phrs.2015.08.003 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7129276/
- Tsumiyama, Ken et al. “Self-organized criticality theory of autoimmunity.” PloS one vol. 4,12 e8382. 31 Dec. 2009, doi:10.1371/journal.pone.0008382 https://pubmed.ncbi.nlm.nih.gov/20046868/
- Shiozawa, Shunichi et al. “DOCK8-expressing T follicular helper cells newly generated beyond self-organized criticality cause systemic lupus erythematosus.” iScience vol. 25,1 103537. 2 Dec. 2021, doi:10.1016/j.isci.2021.103537 https://pubmed.ncbi.nlm.nih.gov/34977502/
- Namekata, Kazuhiko et al. “DOCK8 is expressed in microglia, and it regulates microglial activity during neurodegeneration in murine disease models.” The Journal of biological chemistry vol. 294,36 (2019): 13421-13433. doi:10.1074/jbc.RA119.007645 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6737224/
- Ovsyannikova, Inna G, and Gregory A Poland. “Vaccinomics: current findings, challenges and novel approaches for vaccine development.” The AAPS journal vol. 13,3 (2011): 438-44. doi:10.1208/s12248-011-9281-x https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3160164/
- Galeotti, Caroline et al. “IVIG-mediated effector functions in autoimmune and inflammatory diseases.” International immunology vol. 29,11 (2017): 491-498. doi:10.1093/intimm/dxx039 https://pubmed.ncbi.nlm.nih.gov/28666326/
Insertional Mutagenesis and Cancer (Pt 1)
- The potential for adventitious viral or genomic genetic material found in vaccines to integrate into the DNA of the vaccine recipient is highly concerning and has yet to be sufficiently addressed.
- Incidence of cancers in children has been skyrocketing. Almost no one is talking about the possibility that insertional mutagenesis is causing these cancers as well as causing serious dysregulation of the immune system.
- Retroviruses are known to promote cancer through their ability to insert into host DNA and disrupt the expression of genes involved in growth and differentiation.
- Live viral vaccines were originally grown on animal cells, and they became contaminated with animal retroviruses. It is likely that the growth of the Polio vaccine on mouse cells resulted in the transfer of mouse retroviruses to humans. Further, the introduction of the cancer-promoting monkey virus Simian Virus 40 (SV40) into humans resulted from growing the Polio vaccine in VERO cells (monkey kidney cells lacking anti-viral defenses).
Insertional Mutagenesis and Cancer (Pt 2)
- To resolve the issue of infecting humans with animal retroviruses, vaccinologists decided to grow live viral vaccines on human cells (from aborted fetuses). However, humans also have endogenous retroviruses that can integrate into the genome of the vaccine recipient. Moreover, residual human cell debris present in live viral vaccines contain short strands of DNA and RNA that are easily and preferentially taken up by stem cells in the blood and integrated into the DNA of the vaccine recipient. Integration of viral DNA or short strands of genomic DNA into the DNA of the vaccine recipient will cause insertional mutagenesis, whereby the regulation and expression of normal genes becomes disrupted. If these mutated genes are involved in the regulation of cell growth and division (proto-oncogenes or tumor suppressor genes), then the likely result is cancer, most likely, leukemia or lymphoma.
Dealing with Insertional Mutagenesis
- The potential link between insertional mutagenesis and cancers of the immune system has been almost completely ignored. Clearly, cancer is a very serious diagnosis and this type of dysfunction is among the most difficult to treat. Currently, most people engage in standard allopathic treatments for leukemia and lymphoma, which I will not detail here. In order to correct the dysfunction, stem cell transplants appear to be necessary. First the aberrant/mutated clone of cells must be destroyed, and then these rogue cells can be replaced with unaffected stem cells, often derived from cord blood.
- Research needs to be done to determine if, and at what rates, leukemias and lymphomas are the result of insertional mutagenesis. This would be done by isolating cancerous cells from patients and using molecular techniques (such as PCR) to determine if DNA is integrating into various known “hot spots” in the genomic DNA. The origin of the DNA can also be determined using these methods.
- Healing modalities that reduce the immune activation can also be helpful. These include:
- Healing Leaky Gut
- Shutting down viral re-activation and other infections
- Reducing Inflammation
Key Studies on Insertional Mutagenesis
- Jarzyna, Peter et al. “Insertional mutagenesis and autoimmunity induced disease caused by human fetal and retroviral residual toxins in vaccines.” Issues in law & medicine vol. 31,2 (2016): 221-234. https://pubmed.ncbi.nlm.nih.gov/29108182/
- Fan, Hung, and Chassidy Johnson. “Insertional oncogenesis by non-acute retroviruses: implications for gene therapy.” Viruses vol. 3,4 (2011): 398-422. doi:10.3390/v3040398 https://pubmed.ncbi.nlm.nih.gov/21994739/
- Sokol, Martin et al. “Novel principles of gamma-retroviral insertional transcription activation in murine leukemia virus-induced end-stage tumors.” Retrovirology vol. 11 36. 19 May. 2014, doi:10.1186/1742-4690-11-36 https://pubmed.ncbi.nlm.nih.gov/24886479/
- “The Virus and the Vaccine” article by printed in The Atlantic
- “The Virus and the Vaccine” book by Debbie Bookchin & Jim Schumacher
- “Plague” book by Judy Mikovits
Chronic or Re-Activated Viral Infection
- In some people, acute viral infections subside but do not completely resolve and become a chronic viral infection. Something similar can occur following vaccination with attenuated live viral vaccines, such as the measles, mumps, rubella, chickenpox (varicella) and rotavirus.
- In others, latent viruses, such as Ebstein Barr Virus (EBV), Cytomegalovirus (CMV), other herpes family viruses and various retroviruses can be re-activated in response to immune activation, inflammation, and other stressors.
- Chronic, low-level infections with these viruses then leads to chronic immune activation and associated inflammation. Chronic immune activation can lead to “T-cell exhaustion, where the immune system in not able to properly respond to challenges. Further activation can lead to the development of autoimmunity.
- Activation of viruses and retroviruses are also a problem because they are known to promote cancer through T-cell exhaustion, insertional mutagenesis and other mechanisms.
Dealing with Chronic Viral Infection
- Testing can be done to determine which virus is active and then the viral infection can be targeted with antivirals specific for that family of viruses.
- Healing leaky gut can help lessen the activation of the immune system. If the immune system is able to recover sufficiently, it may be able to resolve the viral infection.
- Some practitioners have found bentaine, trimethylglycine (TMG) and dimethylglycine (DMG) to be helpful in resolving chronic viral infection. This is thought to be due to the ability of these compounds to donate methyl groups which can be used by the body to methylate DNA and thereby help “silence” active proviruses.
Key Studies on Chronic Viral Infection
- Wakefield, A J et al. “Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children.” Lancet (London, England) vol. 351,9103 (1998): 637-41. doi:10.1016/s0140-6736(97)11096-0 https://pubmed.ncbi.nlm.nih.gov/9500320/
- Wild, T F. “Measles virus and chronic infections.” Pathologie-biologie vol. 29,7 (1981): 429-33. https://pubmed.ncbi.nlm.nih.gov/7027150/
- Kerr, Jonathan R. “Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors.” Journal of clinical pathology vol. 72,10 (2019): 651-658. doi:10.1136/jclinpath-2019-205822 https://pubmed.ncbi.nlm.nih.gov/31315893/
- Effros, Rita B. “The silent war of CMV in aging and HIV infection.” Mechanisms of ageing and development vol. 158 (2016): 46-52. doi:10.1016/j.mad.2015.09.003 https://pubmed.ncbi.nlm.nih.gov/26404009/
- Baasch, Sebastian et al. “Cytomegalovirus subverts macrophage identity.” Cell vol. 184,14 (2021): 3774-3793.e25. doi:10.1016/j.cell.2021.05.009 https://pubmed.ncbi.nlm.nih.gov/34115982/
- McLane, Laura M et al. “CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer.” Annual review of immunology vol. 37 (2019): 457-495. doi:10.1146/annurev-immunol-041015-055318 https://pubmed.ncbi.nlm.nih.gov/30676822/
- “Plague” book by Judy Mikovits
Chronic Immune Activation: Leading to Immune Suppression and Autoimmunity
Vaccination is one of many causes of chronic immune activation that can contribute to the development of autoimmunity. When the innate immune system is chronically stimulated, it can lead, paradoxically, to immune suppression and the development of autoimmunity. The mechanisms by which this may be occurring are now known to involve T-cells as well as certain immune receptors, known as Toll-Like Receptors (TLRs). Chronic immune activation results in T- cell dysregulation. T-cells respond to immune activation by destroying virally infected cells and cancerous cells. However, prolonged stimulation of the immune system causes T-cells to stop responding, a condition known as T-cell anergy, or T-cell exhaustion.[4] Once T-cells reach this state, they will no longer properly destroy pathogens or cancerous cells, which leaves the body in an extremely vulnerable state. Moreover, if the immune system continues to be stimulated even after T-cells are in a state of exhaustion, it causes the T-cells to become dysregulated in such a way that they induce the production of antibodies that attack the body’s own tissues.[4]
The hyper-activation or hyper-expression of Toll-Like Receptors (specifically TLR7) have also been shown to play an important role in the development of autoimmunity.[11-13] Research has shown that binding of RNA, RNA/DNA hybrids, and microRNAs to TLRs (specifically TLR7) can stimulate an auto-immune response.[11-13] This is often in response to viruses and/or retroviruses in the body, but theoretically could happen in response to the RNA and/or (contaminating) DNA found in genetic vaccines.[11-13] Thus, chronic immune activation may lead to the development of autoimmune conditions through multiple mechanisms. Further, there are many potential sources of chronic immune activation, including leaky gut, mast cell activation, chronic production of the spike protein, aluminum, and viral reactivation. Reducing chronic immune activation by addressing any of these sources may help in addressing autoimmune conditions.
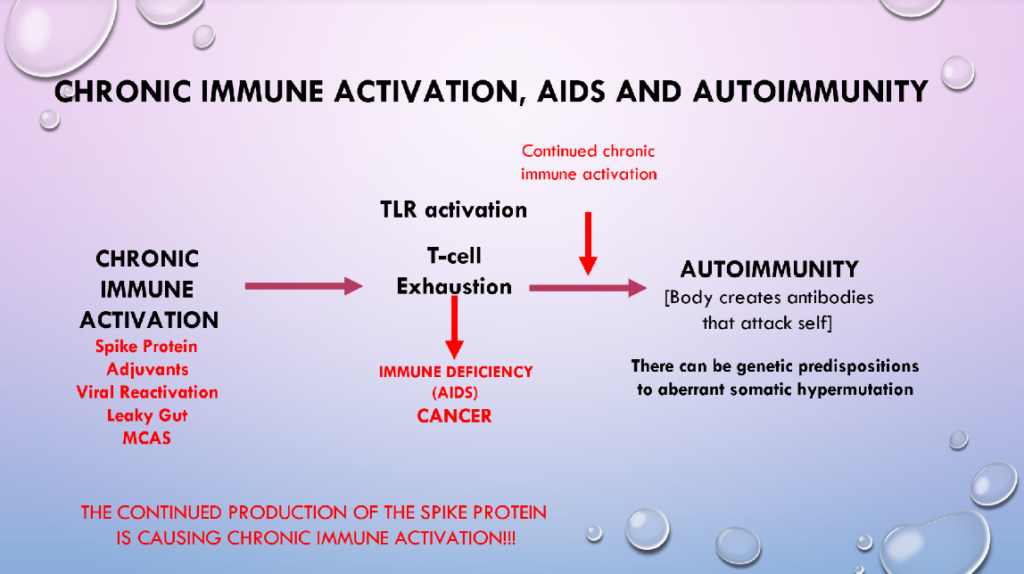
Key Studies on Chronic Immune Activation
- Pennock, Nathan D et al. “T Cell Vaccinology: Beyond the Reflection of Infectious Responses.” Trends in immunology vol. 37,3 (2016): 170-180.doi:10.1016/j.it.2016.01.001 https://pubmed.ncbi.nlm.nih.gov/26830540/
- Guimarães, Luísa Eça et al. “Vaccines, adjuvants and autoimmunity.” Pharmacological research vol. 100 (2015): 190-209. doi:10.1016/j.phrs.2015.08.003 https://pubmed.ncbi.nlm.nih.gov/26275795/
- Föhse, Konstantin et al. “The impact of BNT162b2 mRNA vaccine on adaptive and innate immune responses.” Clinical immunology (Orlando, Fla.) vol. 255 (2023): 109762.doi:10.1016/j.clim.2023.109762 https://pubmed.ncbi.nlm.nih.gov/37673225/
- Tsumiyama, Ken et al. “Self-organized criticality theory of autoimmunity.” PloS one vol.4,12 e8382. 31 Dec. 2009, doi:10.1371/journal.pone.0008382 https://pubmed.ncbi.nlm.nih.gov/20046868/
- Shiozawa, Shunichi et al. “DOCK8-expressing T follicular helper cells newly generated beyond self-organized criticality cause systemic lupus erythematosus.” iScience vol. 25,12 Dec. 2021, doi:10.1016/j.isci.2021.103537 https://pubmed.ncbi.nlm.nih.gov/34977502/
- Holms RD. Long COVID (PASC) Is Maintained by a Self-Sustaining Pro-Inflammatory TLR4/RAGE-Loop of S100A8/A9 > TLR4/RAGE Signalling, Inducing Chronic Expression of IL-1b, IL-6 and TNFa: Anti-Inflammatory Ezrin Peptides as Potential Therapy. Immuno. 2022; 2(3):512-533. https://doi.org/10.3390/immuno2030033
- Baeten, Dominique L P, and Vijay K Kuchroo. “How Cytokine networks fuel inflammation: Interleukin-17 and a tale of two autoimmune diseases.” Nature medicine vol. 19,7 (2013): 824-5. doi:10.1038/nm.3268 https://pubmed.ncbi.nlm.nih.gov/23836225/
- Rose, Noel R. “Negative selection, epitope mimicry and autoimmunity.” Current opinion in immunology vol. 49 (2017): 51-55. Doi:10.1016/j.Coi.2017.08.014 https://pubmed.Ncbi.Nlm.Nih.Gov/29102863/
- Namekata, Kazuhiko et al. “DOCK8 is expressed in microglia, and it regulates microglial activity during neurodegeneration in murine disease models.” The journal of biological chemistry vol. 294,36 (2019): 13421-13433. Doi:10.1074/jbc.Ra119.007645 https://www.Ncbi.Nlm.Nih.Gov/pmc/articles/PMC6737224/
- Ovsyannikova, Inna G, and Gregory A Poland. “Vaccinomics: current findings, challenges and novel approaches for vaccine development.” The AAPS journal vol. 13,3 (2011): 438-Doi:10.1208/s12248-011-9281-x https://www.Ncbi.Nlm.Nih.Gov/pmc/articles/PMC3160164/
- Crampton, Steve P, and Silvia Bolland. “Spontaneous activation of RNA-sensing pathways in autoimmune disease.” Current opinion in immunology vol. 25,6 (2013): 712-doi:10.1016/j.coi.2013.09.011 https://pubmed.ncbi.nlm.nih.gov/24455767/
- Freund, Isabel et al. “RNA Modifications Modulate Activation of Innate Toll-Like Receptors.” Genes vol. 10,2 92. 29 Jan. 2019, doi:10.3390/genes10020092 https://pubmed.ncbi.nlm.nih.gov/30699960/
- von Landenberg, Philipp, and Stefan Bauer. “Nucleic acid recognizing Toll-like receptors and autoimmunity.” Current opinion in immunology vol. 19,6 (2007): 606-10.doi:10.1016/j.coi.2007.10.004 https://pubmed.ncbi.nlm.nih.gov/18060756/
- Krystel-Whittemore, Melissa et al. “Mast Cell: A Multi-Functional Master Cell.” Frontiers in immunology vol. 6 620. 6 Jan. 2016, doi:10.3389/fimmu.2015.00620 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4701915/
Why Join VI-TA?
Practitioner Presentations
Join us twice a month to hear leaders in the field share cutting edge information on what is working to help their patients.
Support on Your Healing Journey
Have information that supports your healing journey at your fingertips when you need it.
Protocols for Healing
Links to the most up-to-date protocols for helping the body recover from vaccine injury and toxic overload.
Learn About Healing Modalities
Learn the pros and cons of different healing modalities and which may be best to support your healing journey.
Become a Member of VI-TA
(for less than the price of 2 cups of coffee a month)

Empowering people with the information they need to maintain and restore health in this age of toxic overload, mass vaccination, and skyrocketing chronic illness.
Contact Us

Empowering people with the information they need to maintain and restore health in this age of toxic overload, mass vaccination, and skyrocketing chronic illness.
Contact Us
- Presentations & Events
- Our Practitioners
- FAQs
- Members Login
- Donate
Become a Member of VI-TA

Empowering people with the information they need to maintain and restore health in this age of toxic overload, mass vaccination, and skyrocketing chronic illness.
Contact Us
- Presentations & Events
- Our Practitioners
- FAQs
- Members Login
- Donate